Punjab State Board PSEB 8th Class Science Book Solutions Chapter 6 Combustion and Flame Textbook Exercise Questions and Answers.
PSEB Solutions for Class 8 Science Chapter 6 Combustion and Flame
PSEB 8th Class Science Guide Combustion and Flame Textbook Questions and Answers
Exercises
Question 1.
List conditions under which combustion can take place.
Answer:
Conditions necessary for combustion:
- Presence of a combustible substance.
- Presence of a supporter of combustion.
- Attainment of ignition temperature.
Question 2.
Fill in the blanks.
(a) Burning of wood and coal causes ……………… of air.
(b) A liquid fuel, used in homes is ………………
(c) Fuel must be heated to its …………………………………… before it starts burning.
(d) Fire produced by oil cannot be controlled by …………………..
Answer:
(a) Pollution.
(b) Kerosene.
(c) Ignition temperature.
(d) Water.
![]()
Question 3.
Explain how the use of CNG in automobiles has reduced pollution in our cities.
Answer:
CNG is a clean gaseous fuel. It burns completely in air and does not produce any harmful gases. So, the use of CNG in automobiles has reduced pollution in our cities.
Question 4.
Compare LPG and wood as fuels.
Answer:
LPG is a clean fuel. It is liquefied petroleum gas and is filled in cylinders. It burns with a blue flame and leaves no residue. Its calorific value is high. Wood is not a clean fuel. It burns and release lots of smoke and ashes are left behind. It give very low amount of heat.
Question 5.
Give reasons:
(a) Water is not used to control fires involving electrical equipment.
(b) LPG is a better domestic fuel than wood.
(c) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not.
Answer:
(a) The substance used for extinguishing fires on electrical appliances or circuit should not be good conductor of electricity as there are chances of electrocution. So, water being a good conductor of electricity, is not used for extinguishing fires on electrical appliances or circuit. Carbon-tetrachloride (non-electrolyte) is used for this purpose.
(b) LPG is liquefied petroleum gas. It has high calorific value of 50 kJ/g. It is neat and clean fuel. It burns with a smokeless flame and does not produce poisonous gases. While wood has less calorific value of 17 kJ/g. It gives out smoke and poisonous gases on burning. So, LPG is better fuel than wood.
(c) When paper wrapped around an aluminium pipe is brought near a flame, it does not burn because the heat gets transferred to aluminium pipe and the ignition temperature of paper is not achieved.
Question 6.
Make a labelled diagram of a candle flame.
Answer:
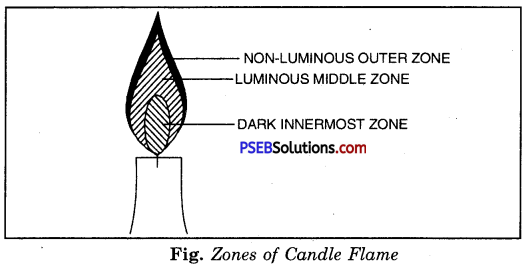
Question 7.
Name the unit in which the calorific value of a fuel is expressed.
Answer:
Kilojoules per kilogram (kJ/kg).
Question 8.
Explain how CO2 is able to control fires.
Answer:
CO2 gas is heavier than air. So, it forms an envelope around the burning fire. This, cuts off the supply of oxygen gas and fire stops burning and gets under control.
Question 9.
It is difficult to burn a heap of green leaves but dry leaves catch fire easily. Explain.
Answer:
Green leaves have a high moisture content in them and in the heap of green leaves, oxygen present is very less. Whereas dry leaves have no moisture content and there is lot of oxygen available in this heap. So, it is difficult to burn a heap of green leaves than a heap of dry leaves.
![]()
Question 10.
Which zone of flame does a goldsmith use for melting gold and silver and why ?
Answer:
Goldsmith usually uses the upper most, non-luminous, blue flame for melting gold and silver because it is the hottest part of the flame.
Question 11.
In an experiment 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 180,000 kJ. Calculate the calorific value of the fuel.
Solution:
Mass of fuel = 4.5 kg
Heat produced = 180,000 kg
Now, Calorific value of a substance = \(=\frac{\text { Heat produced }}{\text { Mass }}\)
= \(\frac{180,000}{4 \cdot 5}\) kj/kg
= \(\frac{40,000}{1}\)
= 4 × 104 kj/kg
Question 12.
Can the process of rusting be called combustion ? Discuss.
Answer:
Rusting.
When iron is exposed to moist air, it gets coated with hydrated iron oxide. This process is called rusting and the coating formed is called rust. Chemically rust is hydrated form of ferric oxide, Fe2O3. xH2O. It is reddish brown in colour.
The overall reaction for rusting is:

Combustion is an oxidation reaction and rusting is also an oxidation reaction but very slow. So, rusting can be termed as slow combustion reaction.
Question 13.
Abida and Ramesh were doing an experiment in which water was to be heated in a beaker. Abida kept the beaker near the wick in the yellow part of the candle flame. Ramesh kept the beaker in the outermost part of the flame. Whose water will get heated in a shorter time.
Answer:
Ramesh’s beaker will get heated in a shorter time because the outermost part of flame is the hottest part.
PSEB Solutions for Class 8 Science Combustion and Flame Important Questions and Answers
Multiple Choice Questions
Question 1.
With the help of the following diagram tell the colour of its flame?
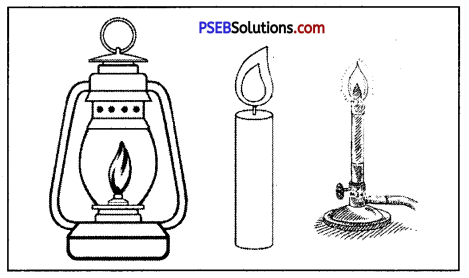
Answer:
The colour of the flame of lamp: yellow
The colour of the flame of candle : yellow
The colour of the flame of bunsen burner : blue.
Question 2.
In the figure below various parts of candle flame are given. Tell which one of them is less hot ?
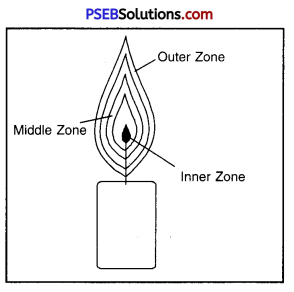
(а) Outer Zone
(б) Inner Zone
(c) Middle Zone
(d) None of these.
Answer:
(c) Middle Zone.
Question 3.
Fuel is:
(a) Solid
(b) Liquid
(e) Gas
(d) Solid, liquid and gas
Answer:
(d) Solid, liquid and gas
Question 4.
Which of the following gas is supporter in combustion?
(a) Oxygen gas
(b) L.P.G.
(e) Nitrogen gas
(d) Carbondioxide gas
Answer:
(a) Oxygen gas.
![]()
Question 5.
Which of the following is combustible substance ?
(a) Iron nail
(b) Glass
(c) Paper
(d) A piece of stone
Answer:
(c) Paper
Question 6.
The conditions for combustion are:
(a) Fuel and air
(b) Fuel, air and ignition temperature
(c) Fuel and ignition temperature
(d) Air and ignition temperature.
Answer:
(b) Fuel, air and ignition temperature.
Question 7.
Which out of the following has minimum ignition temperature ?
(a) Petrol
(b) Kerosene
(c) Coal
(d) L.P.G.
Answer:
(d) L.P.G.
Question 8.
Which of the following gas causes acid rain ?
(a) H2
(b) N2
(c) Carbon-monoxide
(d) Oxides of sulphur and nitrogen
Answer:
(d) Oxides of sulphur and nitrogen.
Question 9.
While extinguishing fire of electric wires and electric instruments, which of the following item is not used ?
(a) Sand
(b) Water
(c) Foam
(d) Carbondioxide
Answer:
(b) Water.
Question 10.
The unit of Calorific value of a fuel is:
(a) Joule
(b) Kilo joule/kg
(c) Kilo joule
(d) None of the above.
Answer:
(b) Kilo joule/kg.
Very Short Answer Type Questions
Question 1.
List the fuels used in rural areas in our country.
Answer:
Wood, agricultural wastes and cow-dung cakes.
![]()
Question 2.
Which of the following solid fuels has the highest heat value: Cow-dung cakes, coal, wood?
Answer:
Coal.
Question 3.
Name the most commonly used liquid fuel in Indian homes.
Answer:
Kerosene.
Question 4.
Name three liquid fuels.
Answer:
Kerosene oil, Petrol, Diesel.
Question 5.
When fuels burn, what do they produce?
Answer:
Heat and light.
Question 6.
What is ignition temperature?
Answer:
Ignition temperature. The lowest temperature at which a substance catches fire in the presence of oxygen, is called its ignition temperature.
Question 7.
Name two liquids which have low ignition temperatures.
Answer:
- Alcohol and
- ether.
Question 8.
What type of fire èxtinguisher is used to extinguish fire caused by
electricity?
Answer:
Carbon tetrachioride (CCl4) fire extinguisher.
![]()
Question 9.
What is combustion ?
Answer:
Combustion.
It is the process of heating of a substance in the presence of oxygen with the evolution of heat and light.
It is an oxidation reaction in which substance (fuel) burns in the presence of oxygen so as to liberate heat and light.
Question 10.
Define heat value of a fuel.
Answer:
Heat value of fuel. The amount of heat liberated when 1 kg of substance is burned, is called heat value of that fuel.
Question 11.
Name the type of fire extinguisher used for extinguishing oil fires.
Answer:
Foam type fire extinguisher.
Question 12.
Why charcoal is considered better fuel than wood ?
Answer:
It is because calorific value of charcoal is higher than that of wood.
Question 13.
Name the zones of a candle flame.
Answer:
- Cold innermost zone,
- middle zone,
- outermost non-luminous zone.
Question 14.
Name two substances which may be effective in fire fighting.
Answer:
Water and Foam.
Question 15.
Name three combustible substances.
Answer:
Paper, wood and cooking gas are three combustible substances.
![]()
Short Answer Type Questions
Question 1.
What are fuels ? In which different states fuels are found?
Answer:
Fuels. The materials which are burnt to produce heat and light, are called
fuels.
Fuels are found in three states:
- Solid (wood, coal, charcoal etc.)
- Liquid (petrol, kerosene, diesel)
- Gases (natural gas, coal gas, bio-gas etc).
Fuels are found in Maharashtra and Gujarat states.
Question 2.
How will you prove that air is required for combustion?
Answer:
A burning coal or wood piece stops burning after some time, if it is covered with a glass vessel. This is because the supply of air is cut off, which stops combustion. If air is blown on this burning piece, it again starts burning with a blaze. It is therefore, concluded that air (supporter of combustion) is required for burning of a substance.
Question 3.
How can accidental fires be extinguished?
Answer:
Accidental fires can be extinguished by controlling any one of the three conditions responsible for producing fire:
- by cutting supply of air (oxygen) so that the combustible substance does not come in contact with the supporter of combustion.
- by cooling the burning substance or by lowering its ignition temperature.
- by removing nearby combustible substances so that fire does not spread.
Question 4.
Why do Goldsmiths use a blow-pipe?
Answer:
Goldsmiths use a blow-pipe to intensify a kerosene lamp flame for moulding pieces of gold or silver into desired shapes. The air blown through the pipe aids combustion of unburnt particles of fuel, thereby making the flame hotter.
Question 5.
Why do combustible substances not catch fire on their own ?
Answer:
Combustible substances cannot catch fire on their own as their ignition temperature is higher than normal temperature. When the temperature is lowered than ignition temperature, they catch fire.
Question 6.
Explain Ignition temperature, combustible substance, supporter of combustion in reference to conditions of combustion.
Answer:
Ignition temperature is the minimum temperature at which fuel catches fire. Each substance has definite ignition temperature below which it will not catch fire.
Combustible substance is that which readily catches fire. Paper, LPG, cloth etc. are combustible substances.
Supporter of combustion is that substance which helps in burning the combustible substance. Combustible substances like petrol, LPG will not burn till supporter of combustion like oxygen is supplied in ample amount.
Question 7.
Given the reaction:
C + O2 → CO2 + 385 kJ
Calculate the calorific value of carbon (atomic weight of C = 12, O = 16).
Solution:
According to equation, 1 mole of carbon or 12 g of carbon on burning produce 385 kJ of heat.
Thus
12 g of carbon produce heat = 385 kJ
1 g of carbon produces heat = \(\frac{385}{12}\)
∴ Calorific value of carbon = 32.1 kJ/g
![]()
Question 8.
Why is a match stick lighted on rubbing it on the rough surface provided on the side of the match box ?
Answer:
This is due to the fact that when we rub the match stick on the rough surface of the match box, the work done during rubbing produces heat due to friction. This heat raises the temperature of the chemical present on the match stick head to its ignition temperature. Therefore, the chemical substance catches fire and the match stick starts burning.
Question 9.
Why is coke considered a better fuel than coal ? Give four reasons.
Answer:
Coke is considered a better fuel than coal because of the following reasons:
- Coke has higher calorific value than coal.
- Ignition temperature of coke is less than that of coal.
- Coke produces less smoke than coal.
- Coal on burning produces some pollutants like CO2, CO and small quantities of SO2 whereas coke does not
produce these pollutants.
Question 10.
What do you mean by fire fighting ?
Answer:
Fire Fighting.
By fire fighting we mean to put out the fire. A fire may be due to an accident, short circuiting or human negligence. The combustion of a substance requires combustible substances, oxygen (or air) and heat. Therefore, to put out fire the air or oxygen supply should be cut off.
Question 11.
What should you do to put out the fire ?
Answer:
A fire may be due to an accident, short circuiting or human negligence. The combustion of a substance requires combustible substance, oxygen and heat. Therefore, to put out fire the air supply should be cut off. The fire can be put out by using carbon dioxide, water, sand etc.
Question 12.
Why should water not be used to extinguish fire due to :
(i) Kerosene or Petrol
(ii) Short circuiting ?
Answer:
(i) This is because kerosene and petrol being lighter than water, float on it and may further spread fire.
(ii) This is because water conducts electricity and it can cause a fatal shock to the person who is putting out the fire.
Question 13.
Why do we wrap a blanket around a person who has caught in lire ?
Answer:
When a person is caught in fire is wrapped with blanket does not get proper supply of oxygen which is necessary for combustion to take place.
Question 14.
Why are we advised not to sleep in a room with a burning coal ingithi in it ?
Answer:
In a closed room there is limited supply of oxygen and due to incomplete combustion of coal, carbon monooxide gas is produced. This gas is poisonous and may prove fatal. So we are advised not to sleep in a closed room with burning coal ingithi in it.
![]()
Question 15.
Explain Global warming.
Answer:
Global Warming. Combustion of most fuels releases carbondioxide in the air resulting in the increase of temperature of the atmosphere which is believed to be the cause of Global warming. Global warming results in melting of polar glaciers causing floods in the low lying coastal areas.
Question 16.
What is acid rain ?
Answer:
Acid Rain.
Burning of coal and diesel releases sulphur dioxide gas which is very pungent and suffocating. Moreover, petrol engines give off oxides of nitrogen. These oxides of sulphur and nitrogen dissolve in water of rain to form acids. Such rain is called Acid rain.
Question 17.
Why does the flame shown in the picture extinguishes when a glass jar is put on the burning candle ?
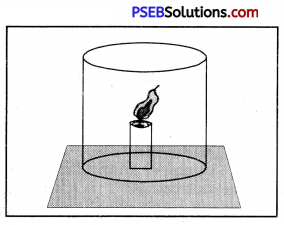
Answer:
We know that oxygen is necessary for burning but when we put a glass jar on the burning candle, the amount of oxygen becomes less. The flame of candle extinguishes because of shortage of oxygen.
Long Answer Type Questions
Question 1.
Discuss the various combustion zones of a candle flame with the help of a diagram.
or
Explain the zones of candle with the help of a diagram.
Answer:
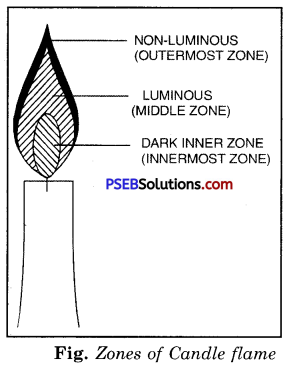
A candle flame has three distinct zones.
1. Dark inner Zone
2. Luminous Zone
3. Non-luminous Zone.
1. Dark inner zone.
The innermost zone is dafk and is the coldest part of the flame. It consists largely of the hot vapours of wax (the combustible material). Introduce one end of a glass tube in the zone, you will see white vapours coming out from the other end of the tube. On being lighted with a matchstick, these emerging vapours burn with a flame.
2. Luminous Zone.
The middle zone is a bright luminous zone. In this zone the fuel partially burns forming carbon particles. It is the glow of these particles that makes this zone luminous. The carbon particles leave the flame as smoke and soot.
3. Non-luminous Zone.
It is the outer zone which is faintly bluish in colour. In this zone oxygen from the air mixes with the fuel bringing about complete combustion. This zone is the outermost non-luminous zone. It has the highest temperature of around 1800°C.
Question 2.
Describe
(i) Rapid combustion
(ii) Spontaneous combustion
(iii) Slow combustion
(iv) Explosion.
Answer:
(i) Rapid Combustion.
The oxidation reaction in which heat and light is produced in a short time is called rapid combustion. For example, when a burning splinter is brought near the gas burner, the gas tap of which is opened, the gas immediately starts burning with the production of heat and light. Similarly, a candle starts burning when a burning splinter is brought close to its wick.
(ii) Spontaneous Combustion.
Combustion which takes place without the application of any external heat is known as spontaneous combustion.
White phosphorus is the best example of a substance burning with spontaneous combustion.
(iii) Slow Combustion.
It is a slow oxidation process in which no light is produced. In such a reaction the heat liberated is at such a low rate that we cannot feel it.
Rusting of iron and the process of respiration are examples of slow combustion.
(iv) Explosion.
Such a process of combustion, where a large number of gases with the evolution of the tremendous amount of heat and light are evolved is called an explosion. During the Diwali festival, we observe that certain crackers explode only when pressure is applied. In this process, the oxidation of the chemicals in the cracker takes place at a very high speed. Large amounts of gases are liberated with the evolution of a tremendous amount of heat and light.
Gunshot, when fired, is called an explosion.
