Punjab State Board PSEB 11th Class Chemistry Important Questions Chapter 8 Redox Reactions Important Questions and Answers.
PSEB 11th Class Chemistry Important Questions Chapter 8 Redox Reactions
Very Short Answer Type Questions
Question 1.
What are spectator ions? Give one example.
Answer:
Spectator ions are ions that stay unaffected during a chemical reaction. They appear both as reactant and as product in an ionic equation. For example, in the following ionic equation, the sodium and nitrate ions are spectator ions.
Ag+ (aq) + NO3–(aq) + Na+ (aq) + Cl– (aq) → AgCl(s) + Na+ (aq) + NO–3 (aq)
![]()
Question 2.
Why is anode called oxidation electrode, whereas cathode is called reduction electrode?
Answer:
At anode, loss of electrons takes place, i.e., oxidation takes place, whereas at cathode, gain of electrons takes place, i.e., reduction takes place.
Therefore, cathode is called reduction electrode and anode is called oxidation electrode.
Question 3.
Can we use KCl as electrolyte in the salt bridge of the cell?
Answer:
KCl cannot be used as electrolyte in the salt bridge because Cl– ions will combine with Ag+ ions to form white precipitates of AgCl.
Question 4.
What would happen if no salt bridge were used in the electrochemical cell (e.g., Zn – Cu cell)?
Answer:
If no salt bridge is used, the positive ions (i.e., Zn2+ ) formed by loss of electrons will accumulate around the zinc electrode and negative ions (i.e., \(\mathrm{SO}_{4}^{2-}\)) left after reduction of Cu2+ ions will accumulate around the copper electrode. Thus, the solution will develop charges and the current stops flowing. Further, since the inner circuit is not complete, the current stops flowing.
Question 5.
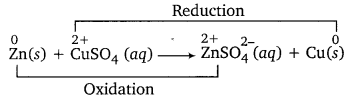
Zn rod is immersed in CUSO4 solution. What will you observe after an hour? Explain your observation in terms of redox reaction.
Answer:
The blue colour of CuSO4 solution will get discharged and reddish brown copper metal will be deposited on Zn rod. This is because blue colour Cu2+ (in CuSO4) gets reduced to Cu by accepting two electrons from Zn, which gets oxidised to colourless ZnSO4.
![]()
Question 6.
What is the most essential conditions that must be satisfied in a redox reaction?
Answer:
In a redox reaction, the total number of electrons lost by the reducing agent must be equal to the number of electrons gained by the oxidising agent.
Question 7.
Find the value of n in \(\mathrm{MnO}_{4}^{-}\) + 8H+ + ne– → Mn2+ + 4H2O
Answer:
\(\mathrm{MnO}_{4}^{-}\) + 8H+ + ne– → Mn2+ + 4H2O
-1 + 8 + n = + 2
-1 – 2 + 8 + n = 0
n = – 5 or 5e–
Question 8.
Can Fe3+ oxidise Br– to Br2 at 1 M concentrations?
\(\boldsymbol{E}^{\ominus}\)(Fe3+ /Fe2+) – 0.77 V and \(\boldsymbol{E}^{\ominus}\)(Br/Br– ) = 1.09 V
Answer:
Es ( Fe3+ / Fe2+) is lower than that of Es(Br / Br–).
Therefore, Fe2+ can reduce Br2 but Br– cannot reduce Fe3+. Thus, Fe3+ cannot oxidise Br– to Br2.
Question 9.
Identify the substance that get reduced in the following reaction:
Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g)
Answer:
In the reaction, Fe2O3 loses oxygen and is reduced to Fe.
Question 10.
Can the following reaction, \(\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}+\mathrm{H}_{2} \mathrm{O} \rightleftharpoons 2 \mathrm{CrO}_{4}^{2-}+2 \mathrm{H}^{+}\) be regarded as a redox reaction?
Answer:
In this reaction, oxidation number of Cr in \(\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}\) is +6 and oxidation number of Cr in \(\mathrm{CrO}_{4}^{2-}\) is +6. Since, during the reaction, the oxidation number of Cr has neither decreased nor increased, therefore, the above reaction is not a redox reaction.
Short Answer Type Questions
Question 1.
2Cu2S + 3O2 ⇌ 2Cu2O + 2SO2
In this reaction which substance is getting oxidised and which substance is getting reduced? Name the reducing agent and oxidising agent.
Answer:
Since, oxygen is being added to Cu, therefore, Cu2S is oxidised to Cu2O and the other reactant i.e., O2 is getting reduced. Hence, Cu2S is a reducing agent and O2 is an oxidising agent.
Question 2.
One mole of N2H4 loses 10 moles electrons to form a new compound Y. Assuming that all the nitrogen appears in the new compound, what is the oxidation number of N in Y? There is no change in oxidation state of H.
Answer:
Suppose the oxidation number of N in Y is x
(N2-)2 → (2N)x + 10e–
(as N2H4 → Y +10e–)
Therefore, 2x -10 = – 4, which gives x = + 3. Hence, oxidation number of N in Y = 3.
![]()
Question 3.
What are the net charges on the left and right side of the following equations? Add electrons as necessary to make each of them balanced half reactions.
(i) \(\mathrm{NO}_{3}^{-}+\mathbf{1 0 H}^{+} \longrightarrow \mathbf{N H}_{4}^{+}+3 \mathrm{H}_{2} \mathrm{O}\)
(ii) \(\mathrm{Cl}_{2}+4 \mathrm{H}_{2} \mathrm{O} \longrightarrow \mathbf{2 C l O}_{2}^{-}+8 \mathrm{H}^{+}\)
Answer:
(i) +9 charge on the left, +1 charge on the right; add 8 electrons to the left side.
(ii) 0 charge on the left, +6 charge on the right; add 6 electrons on the right side.
Question 4.
An iron rod is immersed in solution containing 1.0 M NiSO4 and 1.0 M ZnSO4. Predict giving reasons which of the following reactions is likely to proceed?
(i) Fe reduces Zn2+ ions,
(ii) Iron reduces Ni2+ ions. Given
\(E_{\mathbf{Z n}^{2+} / \mathbf{Z n}}^{\ominus}=-0.76 \mathrm{~V}, E_{\mathrm{Fe}^{2+} / \mathrm{Fe}^{=}}=-0.44 \mathrm{~V}\)
\(E_{\mathrm{Ni}^{2+} / \mathrm{Ni}}^{\ominus}=-0.25 \mathrm{~V}\)
Answer:
(i) Since \(E^{\ominus}\) of Zn is more negative than that of Fe, therefore, Zn will be oxidised to Zn2+ ions while Fe2+ ions will be reduced to Fe. In other words, Fe will not reduced Zn2+ ions.
(ii) Since, \(E^{\ominus}\) of Fe is more negative than that of Ni, therefore, Fe will be oxidised to Fe2+ ions while Ni2+ ions will be reduced to Ni. Thus, Fe reduces Ni2+ ions.
Question 5.
Copper dissolves in dilute nitric acid but not in dilute HC1. Explain.
Answer:
Since, \(E^{\ominus}\) of Cu2+/Cu electrode (+ 0.34 V) is higher than that of H+/H2
electrode (0.0 V), therefore, H+ ions cannot oxidise Cu to Cu2+ ions and hence, Cu does not dissolve in dil. HCl.
In contrast, the electrode potential of \(\mathrm{NO}_{3}^{-}\) ion, i.e.\(\mathrm{NO}_{3}^{-}\) /NO electrode (+0.97 V) is higher than that of copper electrode and hence, it can oxidise Cu to Cu2+ ions and hence Cu dissolves in dil.HNO3 due to oxidation of Cu by \(\mathrm{NO}_{3}^{-}\) ions and not by H+ ions.
Using standard electrode potential, the oxidative and reductive strength of a variety of substances can be composed.
Long Answer Type Questions
Question 1.
Why does fluorine doesn’t show disproportionation reaction?
Answer:
In a disproportionation reaction, the same species is simultaneously oxidised as
well as reduced. Therefore, for such a redox reaction to occur, the reacting species must contain an element which has atleast three oxidation states. The element, in reacting species, is present in an intermediate state while lower and higher oxidation states are available for reduction and oxidation to occur (respectively).
Fluorine is the strongest oxidising agent. It does not show positive oxidation state. That’s why fluorine does not show disproportionation reaction.
![]()
Question 2.
Which method can be used to find out strength of reductant/oxidant in a solution? Explain with an example.
Answer:
Measure the electrode potential of the given species by connecting the redox couple of the given species with standard hydrogen electrode. If it is positive, the electrode of the given species acts as reductant and if it is negative, it acts as an oxidant. Find the electrode potentials of the other given species in the same way, compare the values and determine their comparative strength as an reductant or oxidant.
Examples : Measurement of standard electrode potential of Zn+/Zn electrode using SHE as a reference electrode.
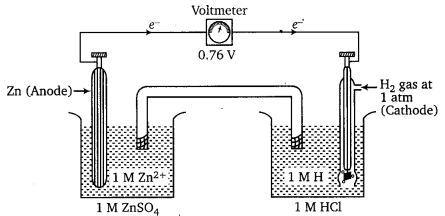
The EMF of the cell comes out to be 0.76 V. (reading of voltmeter is 0.76 V). Zn2+/Zn couple acts as anode and SHE acts as cathode.

