Punjab State Board PSEB 10th Class Science Important Questions Chapter 4 Carbon and its Compounds Important Questions and Answers.
PSEB 10th Class Science Important Questions Chapter 4 Carbon and its Compounds
Long Answer Type Questions
Question 1.
What is allotropy? Name the allotropes of carbon. Are they chemically same? Compare their physical properties.
Answer:
Allotropy: The phenomenon of existence of two or more different physical forms of the same chemical element, with same chemical properties is called allotropy.
Allotropes of carbon
- Diamond
- Graphite
Similarity in chemical composition:
If both the allotropes in equal amount are heated in air then both will release same amount of carbon dioxide and will not leave any residue. Therefore this proves that both are same in chemical composition.
![]()
Comparison of Physical Properties
| Property | Diamond | Graphite |
| 1. Appearance | Transparent | Black, shiny |
| 2. Hardness | Very hard | Soft, slippery/ smooth in touch |
| 3. Heat conductivity | Very less | Medium conductor |
| 4. Electric conductivity | Bad conductor | Good conductor |
| 5. Density (kg/m3) | 3 – 510 | 2 – 250 |
| 6. Purity | Purest | Less pure than diamond |
| 7. Melting point | 3500°C | 3000°C |
| 8. Uses/Applications | Jewellery, drilling purpose Lubricants. | Drycells, Electric Arcs, Pencils, Lead, |
Question 2.
What is covalent bond? Write its properties.
Answer:
The chemical bond formed by sharing of electrons between atoms of same or different non-metal elements is known as covalent bond.
Properties of Covalent bond/bonds.
- They hax e strong bonds within molecules but their inter molecular bond is weak.
- They have low boiling points.
- They have low melting points.
- Their compounds are bad conductors of electricity.
Question 3.
(a) Why does carbon form largest number of compounds?
Answer:
This is due to its tetracovalency and catenation.
(b) Why are some of these called saturated and other unsaturated compounds?
Answer:
The compounds containing C – C single bond are called saturated compounds and the compound containing
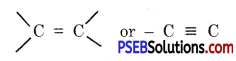
bonds are called unsaturated compounds.
(c) Which of these two is more reactive?
Answer:
Unsaturated compounds are more reactive than saturated compounds.
(d) Write the names of the compounds
(i) CH3 – CH2 – Br
Answer:
![]()
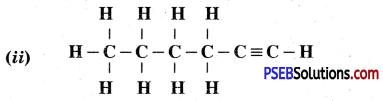
Answer:
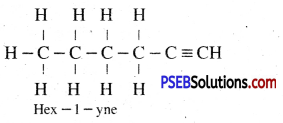
Question 4.
Write about nomenclature of carbon compounds.
Answer:
The names of compounds in a homologous series are based on the name of basic carbon chain modified by a prefix’ ‘phrase before’ or ‘suffix’ ‘phrase after’ indicating the nature of the functional group. For example, the names of various alcohols are methanol, ethanol, propanol and butanol.
Naming of carbon compound can be done by the following method :
- Identify the number of carbon atoms in the compound. A compound having three carbon atoms will have the name propane.
- In case a functional group is present, it is indicated in the name of the compound with either a ‘prefix’ or a ‘suffix’. Like chloro propane, bromo propane, propanal, propanone etc.
- If the name of functional group is to be given on the basis of suffix, the name of the carbon chain is modified by deleting the final ‘e’ and adding the appropriate suffix. For example, a chain having three carbons with a Ketone group will be given name in the following manner.
Propane-‘e’ = propan + ‘one’ = propanone. - If the carbon chain is unsaturated then the final ‘ane’ in the name of carbon chain is substituted by ‘ene’ or ‘yne’. For example, a three-carbon chain with a double bond would be called propene and if it has triple bond, it would be called propyne.
![]()
Question 5.
Write down the chemical properties of carbon compounds.
Answer:
The main chemical properties of carbon compounds are :
1. Burning: Carbons in all its allotropic forms burns in the presence of oxygen and produces heat, light and C02, For example :
C + O2 → CO2 + heat and light
CH4 + 2O2 → CO2 + 2H2O + Heat and light
CH3CH2OH + 3O2 → 2CO2 + 3H2O + Heat and light.
Saturated hydrocarbons burn with a clear flame whereas unsaturated hydrocarbons burn with sooty yellow flame.
2. Oxidation: Carbon compounds can easily be oxidised by burning. Alkaline potassium permanganate or acidified potassium permanganate converts alcohols into acids.
![]()
3. Addition reaction: Unsaturated hydrocarbons make saturated hydrocarbons by adding hydrogen to itself in the presence of catalysts like nickel and palladium. The reaction is commonly used in hydrogenation of vegetable oils using nickel catalyst.
Vegetable oils generally have long unsaturated carbon chain while animal fats have saturated carbon chains.

4. Substitution reaction: Saturated hydrocarbons are fairly unreactive and are inert in the presence of most reagents. However, in the presence of sunlight, chlorine gets combined with hydrocarbons in a quick reaction. Chlorine replaces very quickly the hydrogen atoms in the chemical reaction one by one due to which number of products are usually formed with the higher homologous of alkanes.
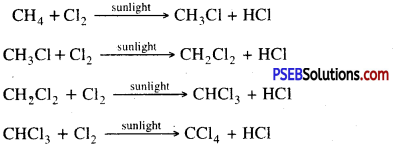
Question 6.
(a) What is an alcohol? Give two examples.
Answer:
Alcohol is a simple compound of carbon, hydrogen and oxygen. If one hydrogen atom is replaced by hydroxyl (-OH) group in alkene, alcohol is produced. General formula of alcohol is CnH2n+2
Examples :
- In Methane (CH4) when hydrogen atom is replaced by hydroxyl (- OH) group, methanol (CH3OH) is formed.
- Ethanol (C2H5OH) is produced with the help of ethane
(b) How is synthetic ethanol produced? Also write.
Answer:
Synthetic ethanol is prepared by reacting ethane with water in the presence of phosphoric acid (H3P04).

Properties of Ethanol :
- It is a colourless liquid with specific colour.
- Its boiling point is 351 K and melting point is 156 K.
- It is soluble in water in any proportion.
- It doesn’t show any reaction with litmus because it is neutral.
- It burns with blue flame in air to produce carbon dioxide and water.
C2H5OH + 3O2 → 2CO2 + 3H2O - It reacts with oxygen or potassium dichromate (K2Cr2O7) to produce ethanoic acid.
C2H5OH + O2 → CH3COOH + H2O - It reacts with sodium metal.
2C2H5OH + 2Na → 2C2H5ONa + H2 - It reacts with acetic acid in the presence of concentrated H2SO4. When this mixture is heated and poured in ice cold solution of sodium carbonate, a sweet smell is produced.
![]()
Question 7.
Give important properties of monocarboxylic acids. Also give its uses.
Or
Give important properties of ethanoic acid (Acetic acid). Also give its uses.
Answer:
Important properties of monocarboxylic acid :
1. Ethanoic acid or acetic acid is colourless liquid and is soluble in water, 5-8% solution of acetic acid in water is called vinegar.
2. Pure ethanoic acid melts at 290 K and freezes during winter in cold climates and looks like ice. Hence it is called glacial acetic acid.
3. Esterification: When an acid is heated with an alcohol in the presence of cone. H2S04, an ester is produced. This is a slow reversible reaction and is called esterification.
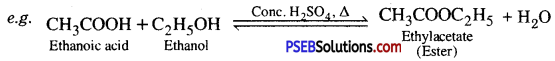
Esters are sweet-smelling substances.
4. Action with a base
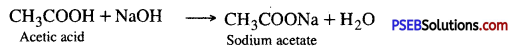
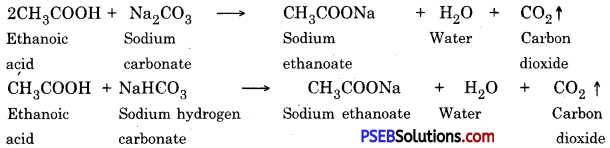
5. Action with carbonates and bicarbonates
Ethanoic acid decomposes carbonates and bicarbonates producing salt, carbon dioxide and water.
2CH3COOH + Na2CO3 → 2CH3COONa + H2O + CO2 ↑
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2 ↑
Uses of ethanoic acid :
- It is used in the manufacture of dyes, perfumes and rayon.
- It is used in the manufacture of plastics, rubber and silk industries.
- It is used as a solvent.
- It is used as a vinegar in cooking, as food dressing and for preparing pickles.
- It is used for the manufacture of chemicals like acetone, acetic anhydride, etc.
- It is used for making white lead [2PbCO3. Pb(OH)2]
Question 8.
Explain the following terms :
(i) Esterification
Answer:
Alcohols are treated with carboxylic acids in the presence of concentrated sulphuric acid to form ester and this process in known as esterification.
Method: Mix ethyl alcohol with acetic acid in a test tube. Few drops of concentrated sulphuric acid is added and test tube is heated mildly in hot tub of water. Instantaneously sweet smell of ester is diffused in whole room.
![]()
It is an example of esterification. Ester is used in ice-cream, cold drinks, medicines, make up etc.
(ii) Saponification
Answer:
Saponification: Breaking down of fats is called saponification.
It is performed by heating vegetable or animal oils with 40% solution of caustic soda. Fats and base react to produce soap and glycerol.
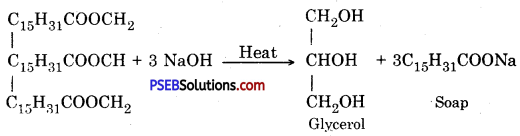
Concentrated common salt solution is added to crystallize soap from water. Soap floats on the surface of water, after cooling. Soap is extracted from water and desired colour and smell are added and is given desired shape.
(iii) Decarboxylation
Answer:
Decarboxylation: Methane is produced when sodium or potassium salt of ethanoic acid are heated with sodium hydroxide and calcium oxide in 3 : 1 ratio.
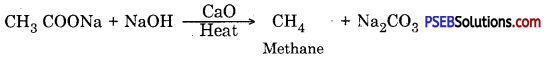
It is a useful method to prepare methane. It is known as decarboxylation because one molecule of CO2 is removed.
(iv) Polymerization.
Answer:
Polymerization: When a large number of small molecules join together at a specific temperature and pressure to form a big molecule, this process is termed as polymerization. These small molecules are known as ‘monomer’ whereas big combined molecule is known as ‘polymer.’
For example. Molecules of ethene join together at 2000 atm pressure and 200°C to form polythene.
![]()
Question 9.
Describe a method for the preparation of soap.
Answer:
Soaps are sodium or potassium salts of higher fatty acids. When the naturally occurring esters called fats or oils are heated with NaOH solution, they undergo hydrolysis to form sodium salt of higher fatty acid (called soap) and glycerol.
Manufacture of Soap. Soap can be made easily in the laboratory. Heat fat or oil with sodium hydroxide solution. After a few minutes, and constant stirring, the oil and water layers get mixed.
Add 5-10 g of common salt to it, stir the mixture and allow’ it to cool. On cooling, pale yellow a solid forms as a cake called soap.
The same principle is used for making soap in soap industry. Some other substances like perfumes, disinfectants and medicines are added to soap to give it desired characteristics.
Example :
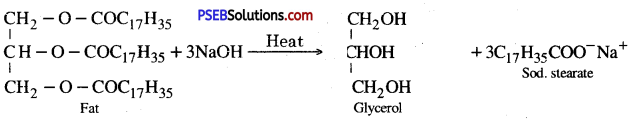
![]()
Question 10.
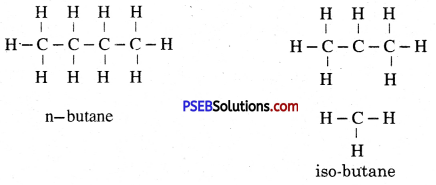
What do you understand by isomers? Explain it with an example.
Or
Write down the isomers of butane.
Answer:
Isomers. The compounds with same molecular formula but different structural formula are called isomers and the phenomenon is called isomerism. There is no change in the structure of methane, ethane, propane if the atoms of carbon and hydrogen are rearranged but in case of alkane molecule if number of carbon is more than three then more than one arrangement is possible.
In this one long carbon chain is formed and the others are branched. In case of butane the chain is linked with atleast three carbon atoms with other carbon atoms. This type of alkanes are termed as iso-alkane. In a chain having no branch, carbon atom cannot be linked with two carbon atoms. Alkanes of this type are called normal alkane (n-alkane).
Two Isomers of Butane:
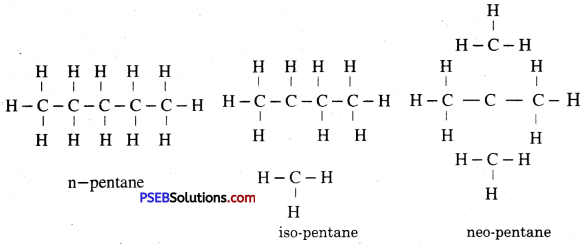
Isomers of Pentane
Short Answer Type Questions
Question 1.
Explain why carbon forms mostly covalent compounds?
Answer:
Carbon has four electrons in its outermost shell. In order to get stable electronic configuration, it has to lose or gain four electrons, but it is difficult to lose or gain four electrons from energy considerations. Hence, Carbon complete its octet by sharing its valence electrons with other atoms forming covatent bonds resulting in the formation of covalent compounds.
Question 2.
In order to form large amount of carbonic compounds which major elements are used other than hydrogen and oxygen?
Answer:
The number of carbonic compounds is very large which was not possible only with the help of hydrogen and oxygen. Along with these two elements some other elements like nitrogen, sulphur, oxygen, halogens (Cl, Br, I, F) etc. also combine and because of it the caybonic compounds have crossed the number of many million. These combine together on the basis of functional groups and make new compounds.
Question 3.
‘Carbon is a unique element’, why?
Answer:
Carbon is the only known element out of all other known elements, that has got capability to make long chains. Each long chain of carbon atoms provides such a simple base with which other atoms can join by various methods resulting in the composition of various compounds.
Carbon atoms can make long chains or rings in three ways because of having catenation property.
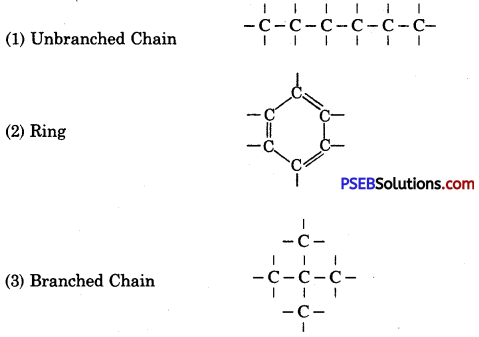
Question 4.
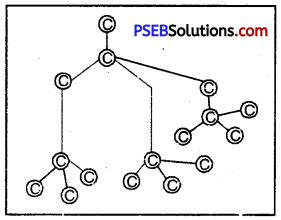
Explain the structure of diamond and write why is it so hard?
Answer:
In diamond each carbon atom remains at the centre of regular tetrahedral and it remains bound with covalent bond to four carbon atoms which are situated at four corners of tetrahedral. Thus, all electrons of carbon are bound atoms and none is found in free state. So it forms a strong three-dimensional tetrahedral structure and because of it diamond is the hardest substance with very high density.
Question 5.
What are the uses of diamond?
Answer:
Uses of Diamond
- Diamond being the hardest substance is used for cutting other substances.
- Diamond because of its shine and lustre is used for making ornaments.
- It is used for drilling hard rocks.
- Sharp-edged diamonds are used by eye surgeon in catract surgery.
- Being extra ordinarly sensitive for heat radiations and having property to remove immediately harmful radiations. These are used in making absolute thermometers.
Question 6.
Why do diamonds glitter?
Answer:
Diamond is a transparent substance whose refractive index is very high. The rays of light passing through it get diverted much. According to its cut base edges light rays undergo total internal reflection. When these edges are polished, diamond glitters very beautifully.
![]()
Question 7.
How are diamonds made naturally and artifically?
Answer:
Naturally, diamonds are made by carbon present at the depth of about 150 km, where pressure is 70,000 atm and the temperature is about 1500°C. These diamonds come up near the surface with special kimberlite rocks. Artifically, diamonds are made by heating graphite under high pressure in the presence of special catalysts.
Question 8.
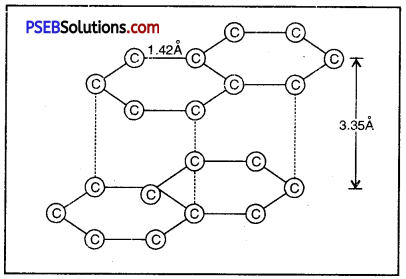
Write the structure of graphite. Why is it so soft?
Answer:
Each carbon atom is bonded with three neighbouring carbon atoms with covalent bonds in graphite. They are always in the same plane giving hexagonal array. As compared to diamond, the distance between carbon atoms is more. The distance between upper and lower layers does not permit carbon atoms to make covalent bonds and because of this fourth electrons remains free. These layers can easily slide one upon the other and graphite attains the property of a lubricant. It is very soft and slippery.
Question 9.
Why are diamonds used in jewellery? Give reasons.
Answer:
Refractive index of diamond is 2.45 which is the highest of all known substances, because of this, it shines brightly. Its critical angle is 24°, which is very less. This is why, the light rays passing through it deviates much. A small deviation in it causes beautiful spectrum of colours. When the bases of diamond are polished, it produces an astonishing brightness. That is why diamonds are used in making jewellery.
Question 10.
Why is graphite a good conductor of electricity?
Answer:
Each carbon atom of graphite is surrounded by three carbon atoms joined with covalent bond which makes hexagonal layers over each other. In these layers distance between the carbon atoms is more, that is why the possibility of fourth covalent bond between the carbon atoms of opposite layers is reduced. The fourth covalent electron is set free. The graphite has easy flow of electrons and it acts as a good conductor of electricity.
Question 11.
What are the uses of graphite?
Answer:
Uses of Graphite
- It is good conductor of electricity so- it is used in making dry cells and electric arcs as electrods.
- It is used in making pencils, black colour and black paint.
- It has lubricant property so it is used in machines to keep them lubricating at high temperature.
- It is used in making crucibles to boil some metals because of its high melting point.
Question 12.
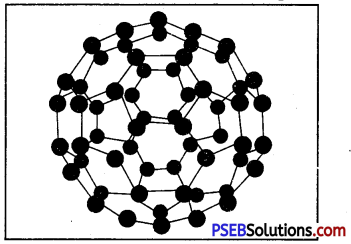
Why are allotropes of carbon in form a molecule known as fullerenes?
Answer:
The allotropes of carbon in which 60 carbon atoms join to form a molecule are termed as fullerene. US architect Buckminster Fuller designed three dimensional domes which were supported by pentagonal and hexagonal structures.
Since fullerene molecules looked like these domes, that is why they are known as fullerene.
Question 13.
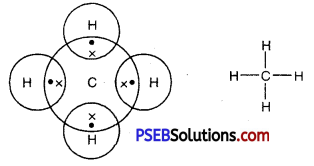
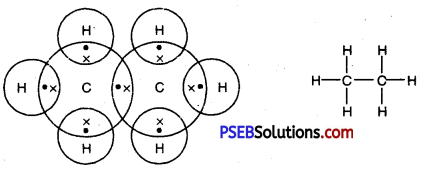
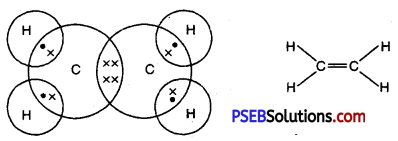
Draw the electron dot structures of methane, ethane, ethene, butane.
Or
(i) Write molecular formula of butane.
(ii) Draw the structure of Propanal.
Answer:
Methane
Formula -CH4
Ethane
Formula -C2H6
Ethene
Formula -C2H4
Or
(i) Butane
Formula – C4H10
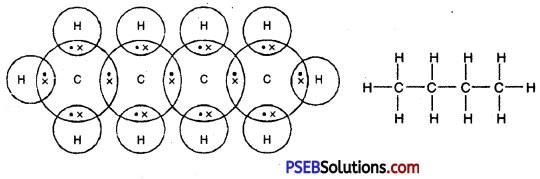
(ii) Propanal
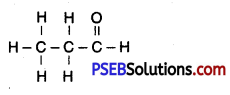
![]()
Question 14.
Write two tests of alcohol.
Answer:
- Ester test: When alcohol is heated with acetic acid, in the presence of sulphuric acid, sweet smelling ester is produced.
- Sodium test: Alcohol reacts with sodium metal and always produces hydrogen gas.
Question 15.
(a) What do you understand by homologous series? Explain with an example.
Answer:
Homologous series. A series of compounds in which the same functional group substitutes for hydrogen in a carbon chain is called a homologous series. Each successive member differ by (- CH2) group. All the members of a homologous series can be obtained by similar process.
General Formula : CnH2n+2
For example : Homologous series of alkane is as follows
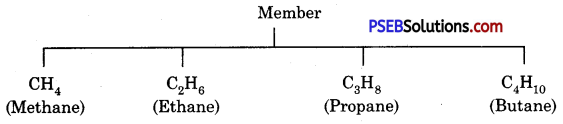
(b) Write the first three homologous compounds of methane.
Answer:
Homologous Compounds of Methane
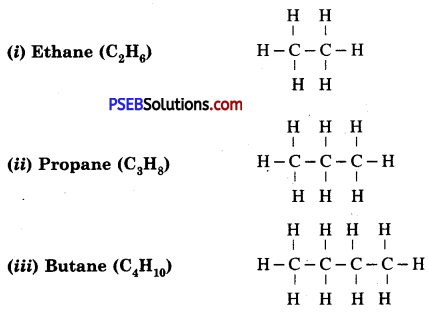
Question 16.
Define homologous series. What are its main properties?
Answer:
Homologous series. A series of compounds in which the same functional group substitutes for hydrogen in a carbon chain is called homologous series. In this series the adjacent members differ by (-CH2) group. Each member of a homologous series is called homolog.
Properties of Homologous Series :
- All the members of a homologous series can be represented by a common formula.
- Two successive members differ by one carbon atom and 2 hydrogen atoms.
- All the members of a homologous series possess similar chemical properties.
- There is a small difference between all the members of a homologous series.
- There is always a difference of 14 a.m.u. in atomic mass of two adjacent homologous.
Question 17.
What are alkanes? Write the properties of its main members. What do we conclude from its properties?
Answer:
Alkanes. The hydrocarbons with structural/general formula CnH2n+2 are called alkanes. The members of this group form an homologous series. Hydrocarbons like methane, ethane, propane and butane form many carbonic compounds which are collectively known as alkane. In these compounds, the number of carbon and hydrogen atoms differ by (- CH2) for the next member of family.
Properties of Alkanes
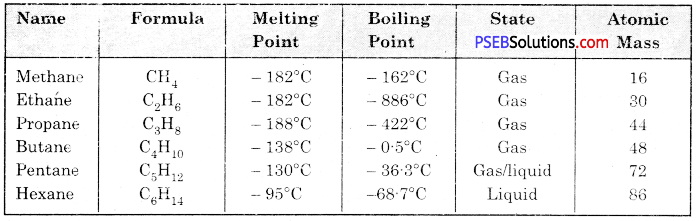
Conclusions:
- Physical properties of alkane are based on their mass.
- First four members of alkane are in gasous state and alkanes having more than six carbon atoms are in liquid state.
- Alkanes having high molecular mass are found in solid state.
- Melting and boiling point of alkanes increase with increase in molecular mass.
Question 18.
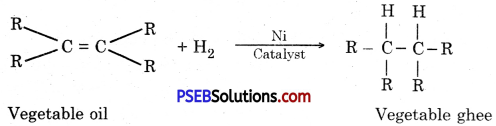
What happens when hydrogen gas is passed through vegetable oils in the presence of nickel?
Answer:
Vegetables oils have double bond and polymerisation is possible. When nickel is used as catalyst, vegetable oil converts to vegetable ghee when hydrogen is passed through it because of hydrogenation. Vegetable ghee is solid like butter at room temperature.
Question 19.
Write the chemical properties of ethanoic acid.
Answer:
Ethanoic acid reacts with some metals, metal carbonates, hydroxides and bicarbonates.
1. Action with sodium:
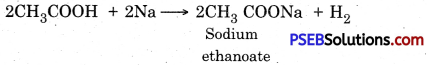
2. Action with sodium hydroxide and potassium hydroxide:
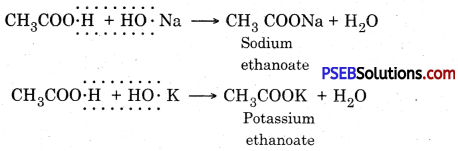
3. Action with carbonate and hydrogen carbonate :
It reacts with carbonates and hydrogen carbonate to form water, salt and carbon dioxide.
![]()
Question 20.
Which type of oils should be used for cooking? Why?
Answer:
Animal fats have saturated fatty acids, which are harmful for health, whereas oils have long unsaturated carbon chains which are not harmful. Hence unsaturated fatty acids oils are used for cooking.
Question 21.
Write the ill-effects of using alcohol.
Answer:
- It is an addictive substance.
- It affects the sensitivity of nervous system.
- When a drunken person drives the vehicle he/she is not able to take the right decisions which leads to accident.
Question 22.
Why is consumption of methanol (CH3OH) very fatal?
Answer:
Even a very small amount of methanol can prove fatal. In liver, it oxides to form methanale, which vigorously react with cells of liver, by which protoplasm gets emanating and swells in the same manner as boiled egg. Consumption of methanol also lead to blindness.
Question 23.
Write the IUPAC names of ethyl alcohol and acetic acid. Write the name of products formed when ethyl alcohol and acetic acid react in the presence of concentrated sulphuric acid. Write its chemical reaction.
Answer:
| Compounds | IUPAC Names |
| 1. Ethyl alcohol | ethanol |
| 2. Acetic acid | ethanoic acid |
Chemical reaction :
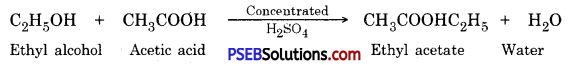
Question 24.
How will you prepare :
(a) ethyl alcohol from ethene
(b) acetic acid from ethyl alcohol?
Give reactions.
Answer:
(a) Ethene is heated with water in the presence of phosphoric acid to prepare ethyl alcohol
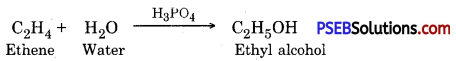
(b) Acetic acid is prepared by oxidation of ethyl alcohol
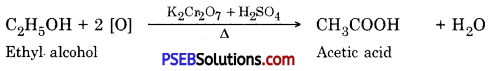
Question 25.
Write in brief the various industrial alcohols.
Answer:
- Absolute alcohol: It is 100% pure ethanol. It is produced by fractional distillation of ethanol which is prepared by fermentation of carbohydrates.
- Denatured alcohol: It is 95% ethanol and is used in industries. It is made poisonous by adding methanol, copper sulphate and pyridine, so as to make it unfit for drinking.
- Power alcohol: It is mixture of Benzene and Ether in 20% Ethanol and 50% gasolene. It is used to save petroleum.
- Alcoholic beverages: Alcohol is used as addictive drink. It is sold under various names like, rum, whisky, brandy, Jinn, etc.
Question 26.
Why is cleansing effect of soap not effective in hard water?
Answer:
Soaps are not effective in hard water for washing purposes because hard water has salts of calaium and magnesium. When soaps are dissolved in hard water, calcium and magnesium ions l’eact with water to form calcium and magnesium salts of fatty acids.
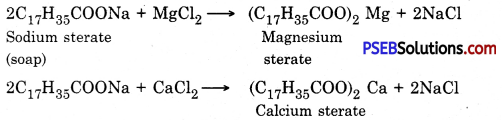
That is why a large amount of soap gets wasted. Calcium and magnesium salts being insoluble in water make precipitates and they get attached with the clothes and cause hinderance to the cleaning action and the clothes are not cleaned properly.
Question 27.
Distinguish between soaps and detergents.
Answer:
| Soaps | Synthetic detergents |
| 1. Soaps are sodium or potassium salts of higher fatty acids e.g. Sodium Stearate. | 1. Synthetic detergents are Sodium alkyl Sulphates or Sodium alkyl benzene sulphates. |
| 2. Soaps are prepared from vegetable oils or animal fats. | 2. Synthetic detergents are prepared from the hydrocarbons obtained from petroleum. |
| 3. Soaps have relatively weak cleansing action. | 3. They have strong cleansing action. |
| 4. Soaps form curdy white precipitates with calcium and magnesium salts present in hard water and hence, are not used in hard water. | 4. Calcium and magnesium salts of detergents are soluble in water. Therefore no curdy white precipitates are obtained in hard water and hence synthetic detergents can be used even in hard water. |
| 5. Soaps cannot be used in acidic medium as they are decomposed into carboxylic acids in acidic medium. | 5. They can be used in acidic medium as they are the salts of strong acid and are not decomposed in acidic medium. |
| 6. Soaps do not cause water pollution. | 6. Synthetic detergents cause water pollution. |
| 7. Soaps are biodegradable. | 7. Some of the synthetic detergents are non biodegradable. |
Question 28.
Write the formation of ethanol by fermentation process.
Answer:
Fermentation occurs in the presence of Bio-chemical catalysts at normal temperature by which sugar molecules convert into alcohol and carbon dioxide. These catalysts are known as enzymes, which means ‘yeast’ or ‘in the fermentation’.
Alcohol is formed by fermentation of sugar or starch. In a beaker, grape juice or glucose mixture is heated at 20° – 30°C in the presence of yeast. Sugar or starch molecules break into smaller molecules which produce carbon dioxide due to process of fermentation. This carbon dioxide is expelled out but air is not given inlet. A dilute mixture of ethanol is formed in water during fermentation. Ethanol is purified by distillation.
Chemical reaction :
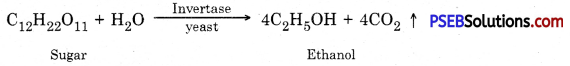
Question 29.
Write the name of the reaction in which ethanoic acid and ethanol react together to form one product. Write the common name of the product.
Answer:
When ethanoic acid reacts with ethanol in the presence of concentrated sulphuric acid, ester is produced and this process is known as esterification.
CH3COOH + C2H5OH → CH3COOC2H5 + H2O
Question 30.
Observe the figure given below :
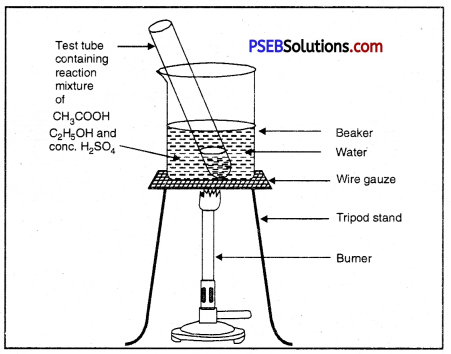
(i) Write the reaction taking place
Answer:
![]()
(ii) What is this reaction called?
Answer:
Esterification
Question 31.
What is indicated in the figure given below and give use of it.
Answer:
The figure indicates formation of micelle. Micelles are used to remove oily dirt from clothes.
1 – Hydrophilic end
2 – Oil droplet.
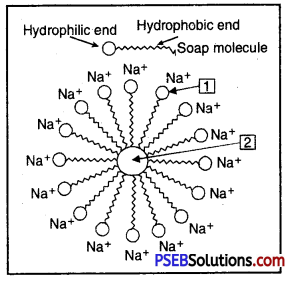
Question 32.
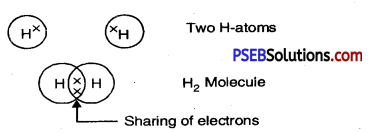
Show the bonds of Hydrogen, Oxygen and Nitrogen atoms respectively.
Answer:
1. Hydrogen: Two atoms of hydrogen by sharing of their electrons make hydrogen molecule (H2).
2. Oxygen: A double bond is made between two atoms of oxygen. Each atom of oxygen shares two electrons with other atom to form .an octet.
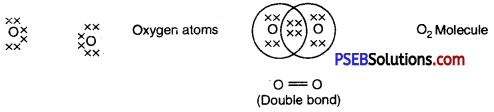
3. Nitrogen: Each atom of nitrogen shares three electrons to form an octet.
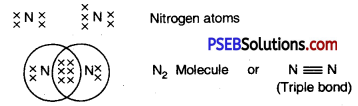
Question 33.
(i) Write molecular formula of Propane.
Answer:
C3H8
(ii) Write IUPAC name of
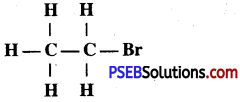
Answer:
Bromoethane.
Question 34.
What is detergent? Write the formation of synthetic detergents. Write its advantage.
Answer:
Materials used for cleaning purposes are known as detergents. Since long, soaps have been used as detergents but now-a-days synthetic detergents are more popular. The detergent molecule has two ends, one which is made of sulphate (-SO4) or sulphonate (SO3Na) group and it is hydrophilic by nature whereas other which is made of hydrocarbon is hydrophobic.
Synthetic detergent produces ample amount of lather even in hard water. These do not form insoluble salts of calcium or magnesium.
Question 35.
Which substances are used to produce synthetic detergents?
Answer:
Following substances are needed for production of synthetic detergents :
- Long chained hydrocarbons obtained from petroleum and coal.
- Concentrated sulphuric acid.
- Sodium hydroxide.
Procedure: Hydrocarbons are treated with cone, sulphuric acid to get hydrocarbonic sulphuric acid. It is further reacted with NaOH which forms synthetic detergents. About 15 to 30% of the total weight of washing powder is detergent. Rest of the others are various chemicals which provide other qualities.
![]()
Question 36.
What are the components of washing powder?
Answer:
- Synthetic detergents (15 to 30%).
- Sodium sulphate and sodium nitrate.
- Sodium tri-poly phosphate or sodium carbonate.
- Sodium perborate.
Very Short Answer Type Questions
Question 1.
Which element is present necessarily in the following items?
Food, clothes, medicines and hooks.
Answer:
Carbon.
Question 2.
On which element do all living processes depend?
Answer:
Carbon.
Question 3.
What percentage of carbon is present in minerals found under the crust of earth?
Answer:
0.02 %.
Question 4.
What percentage of CO2 is present in atmosphere?
Answer:
0.003%.
Question 5.
How many electrons are present in the outer shell of carbon?
Answer:
4.
Question 6.
Why does carbon need four electrons to gain or lose?
Answer:
To attain noble gas configuration.
Question 7.
How does carbon gain four electrons?
Answer:
By making C4– anion.
Question 8.
How does carbon lose four electrons?
Answer:
By making C4+ cation.
Question 9.
Which type of bond is shared by a pair of two hydrogen atoms?
Answer:
Single bond.
Question 10.
How is single bond between two atoms expressed?
Answer:
By single straight line.
Question 11.
What is the atomic number of nitrogen?
Answer:
7.
Question 12.
How many electrons are given by each atom of Nitrogen to complete the octet?
Answer:
3.
![]()
Question 13.
Which gas is used as a fuel the most?
Answer:
Methane gas.
Question 14.
Methane is the main constituent gas of which two fuel gases?
Answer:
Biogas, C.N.G.
Question 15.
Write the full form of C.N.G.
Answer:
Compressed Natural Gas.
Question 16.
How is the structure of diamond?
Answer:
Strong three-dimensional structure.
Question 17.
How is the structure of graphite?
Answer:
Hexagonal.
Question 18.
Which of these two is a good conductor of electricity diamond or graphite?
Answer:
Graphite.
Question 19.
Which is the hardest substance?
Answer:
Diamond.
Question 20.
How does it feel by touching graphite?
Answer:
Soft and slippery.
Question 21.
Which element has the ability to form large number of compounds?
Answer:
Carbon.
Question 22.
Give names of two elements which are placed in the group of carbon and have same valency.
Answer:
Valency of carbon is 4. This group has silicon and germanium.
Question 23.
What is the simplest form of hydrocarbon?
Answer:
Methane (CH4).
Question 24.
In which types of bonds can carbon atoms join?
Answer:
Single, double, triple bond and ring-shaped.
Question 25.
Write the names of five elements which can make bond with carbon to form new compounds.
Answer:
Oxygen, Hydrogen, Nitrogen, Sulphur, Chlorine.
![]()
Question 26.
Write the formula of Ethane.
Answer:
C2H6.
Question 27.
Write the structural formula of Ethane.
Answer:
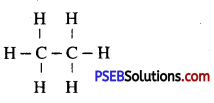
Question 28.
Give the next higher homo-logues of:
(i) C3H6
Answer:
C4H8
(ii) C6H8.
Answer:
C7H10.
Question 29.
Give the structure of ethanol.
Answer:
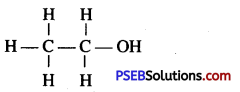
Question 30.
Name the functional group in propanone.
Answer:
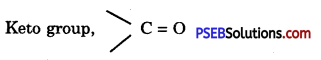
Question 31.
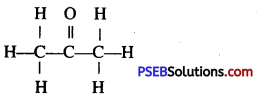
Give the structure of simplest ketone.
Answer:
Simplest keton is acetone. It has the structure :
Question 32.
Give the name and structure of four carbon atoms in aldehyde.
Answer:
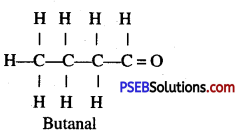
Question 33.
Name the products obtained when ethanol undergoes complete combustion.
Answer:
Carbon dioxide (CO2) and Water (H2O).
Question 34.
Give the structural formula of ethanol.
Answer:
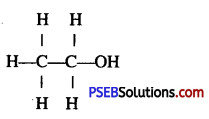
Question 35.
Give the name and structure of functional group present in acetic acid (CH3COOH).
Answer:
Carboxyl group
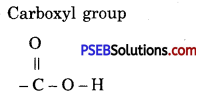
Question 36.
Which of the following compounds belong to same homo-Iogous series?
C2H6O2, C2H6O2, C2H6, CH4O
Answer:
C2H6 O, (C2H5OH) And CH4O or (CH2OH).
Question 37.
Give the structural formula of an ester.
Answer:
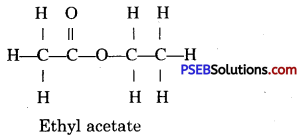
Question 38.
Give the names and structural formulas of acid and alcohol from which ethyl acetate is obtained.
Answer:
Acetic acid
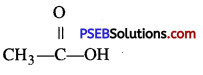
and ethyl alcohol, CH3CH2OH
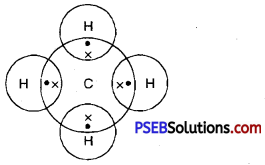
Question 39.
Give the electron-dot stucture of methane.
Answer:
Question 40.
Define covalent bond.
Answer:
It is formed by mutual sharing of electron pair between two atoms.
Question 41.
Explain what covalent compounds do not conduct electric current?
Answer:
This is because there are neither free ions nor free electrons.
Question 42.
Name the bond formed between metal atom and non-metal atoms.
Answer:
Ionic or electro valent bond.
Question 43.
What are allotropes?
Answer:
When an element exists in two or more form having different physical pruperties but, some chemical properties, there forms are called allotropes.
Question 44.
Define catenation.
Answer:
It is the property due to which a large number of atoms of the same element get linked together through covalent bonds forming long straight chains, branched chains and rings of different sizes.
![]()
Question 45.
Give two oxidising agents.
Answer:
- Acidified potassium permanganate.
- Acidified potassium dichromate.
Question 46.
Give the electron dot structure of ethane.
Answer:
Dot structure of ethane :
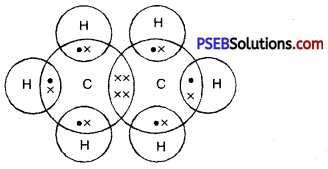
Question 47.
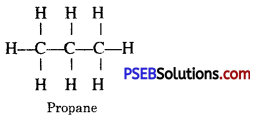
Give the structural formula of propane.
Answer:
Question 48.
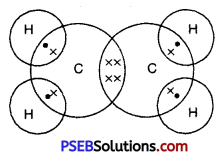
Give the electron dot structure of ethene.
Answer:
Dot structure of ethene.
Question 49.
Define functional group.
Answer:
It is an atom or group of atoms which when present in a molecule gives special properties to it. e.g. C2H5OH has the functional group, -OH (hydroxyl group).
Question 50.
If two organic compounds have some functional group, predict they have similar physical or chemical properties.
Answer:
Similar chemical properties.
Question 51.
Give the general formulae of alkanes, alkenes and alkynes.
Answer:
Alkanes: Cn H2n+2
Alkaenes: CnH2n
Alkynes: CnH2n-2, where n = No. if carbon atoms).
Question 52.
Give the structural formula of isomers of C4H10 (butane).
Answer:
Butane has two structural isomers :
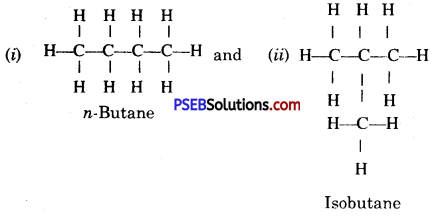
Question 53.
Give the first four members of homologous series of alcohols.
Answer:
CH3OH, C2H5OH. C3H7OH, C4H5OH.
Question 54.
Define a Catalyst.
Answer:
It is a substance which can increase the rate of a reaction but remains unchanged in mass and composition at the end of reaction.
Question 55.
What happens when a piece of sodium metal is added to ethanol? Give chemical equation also.
Answer:
![]()
Question 56.
What happens when ethanol is heated with conc. H2SO4 at 443K?
Answer:
Ethanol undergoes acidic dehydration to give ethene
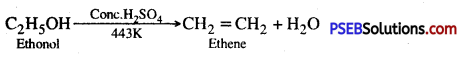
Question 57.
What is denatured spirit or alcohol?
Answer:
It is prepared by adding poisonous substance like methanol, acetone, pyridine or copper sulphate to ethanol. It is unfit for drinking purposes.
Question 58.
Give three uses of ethanoic acid.
Answer:
- In the manufacture of vineger
- In the manufacture white lead
- As a reagent in laboratory.
Question 59.
What is the atomic number of hydrogen?
Answer:
One.
Question 60.
Give the electronic configuration of carbon atom.
Answer:
(2, 4).
Question 61.
Give name of four carbon compounds.
Answer:
Methane, Chloroform, Ethanol and ethanoic acid.
Question 62.
Give two allotropic forms of carbon.
Answer:
Diamond and graphite.
Question 63.
Give the name of simplest ketone.
Answer:
Propanone (CH3 COCH3).
![]()
Question 64.
Give the name of first member of homologous series of alkynes.
Answer:
Ethyne (C2H2).
Question 65.
Give the name and formula of acid present in vinegar.
Answer:
Ethanoic acid (CH3 COOH).
Multiple Choice Questions:
Question 1.
Ethanoic acid has the formula:
(A) C2H5OH
(B) CH3COCH3
(C) CH3COOH
(D) C2H5COOH.
Answer:
(C) CH3COOH
Question 2.
Carboxylic acids contain functional group:
(A) -CHO
(B) -CH2OH
(C) -COOH
(D) -OH.
Answer:
(C) -COOH
Question 3.
The general formula of the alkyne is:
(A) CnH2n-2
(B) CnH2n+2
(C) CnH2n
(D) CnH2n+2.
Answer:
(A) CnH2n-2
Question 4.
Propanone has the functional group :
(A) -OH
(B) -CHO
(C) C=O
(D) -COOH.
Answer:
(C) C=O
Question 5.
Vinegar contains acetic acid:
(A) 5-8%
(B) 15-20%
(C) 21-29%
(D) 30-40%.
Answer:
(A) 5-8%
Question 6.
The reaction of acetic acid with ethyl alcohol is called :
(A) Polymerisation
(B) Saponification
(C) Hydrogenation
(D) Esterification.
Answer:
(D) Esterification.
Fill in the Blanks :
Question 1.
The functional group present in ethyl alcohol is ___________
Answer:
The functional group present in ethyl alcohol is alcoholic group (-OH)
Question 2.
CH3—CH2—OH represents ___________
Answer:
Ethyl alcohol.
Question 3.
Two atoms of the same element combine to form a molecule. The bond between them is known as ___________ bond.
Answer:
Covalent.
![]()
Question 4.
In the formation of oxygen molecule, the oxygen atoms share ___________ electrons each.
Answer:
Two.
Question 5.
The number of single covalent bonds in the molecule of ammonia is ___________
Answer:
Three.
