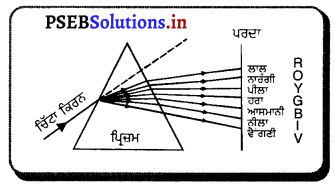
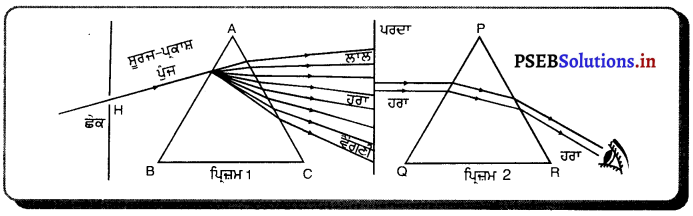
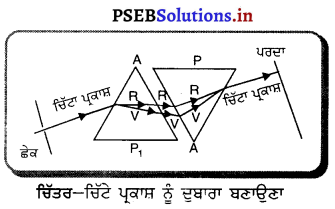
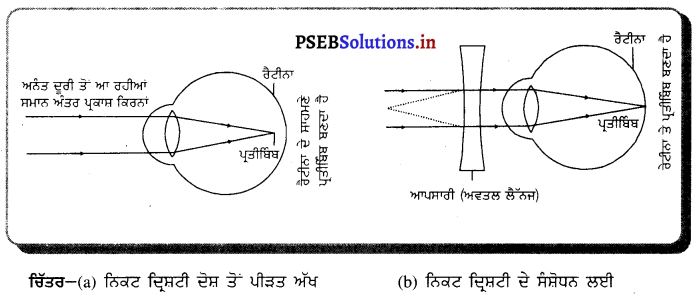
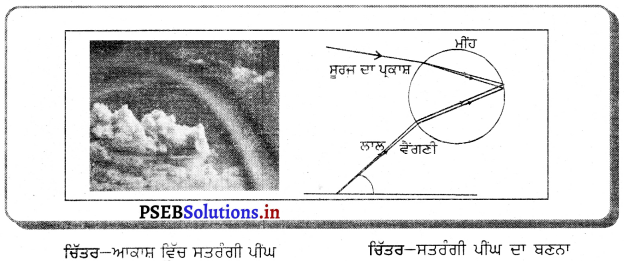
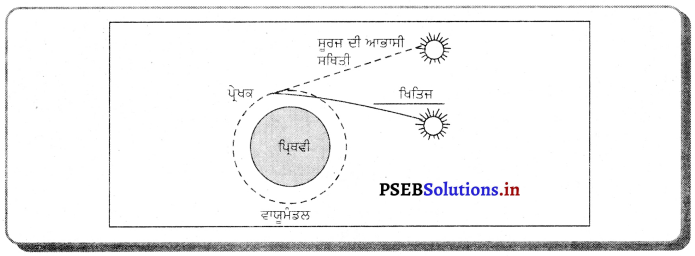
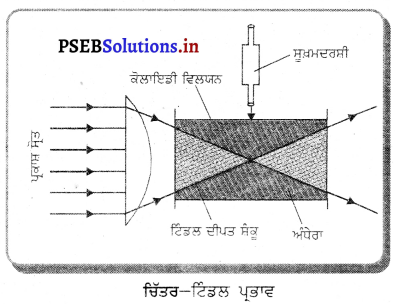
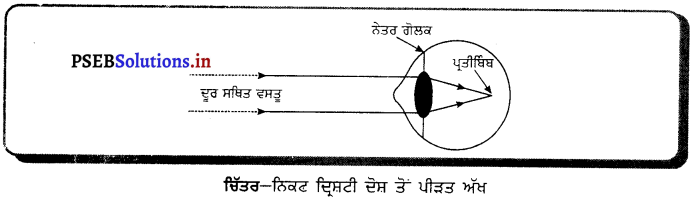
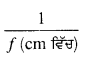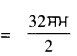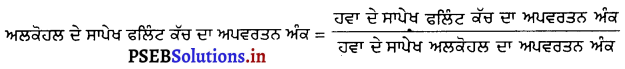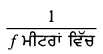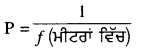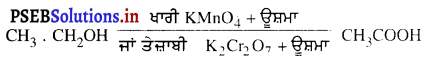Punjab State Board PSEB 10th Class Science Important Questions Chapter 10 ਪ੍ਰਕਾਸ਼-ਪਰਾਵਰਤਨ ਅਤੇ ਅਪਵਰਤਨ Important Questions and Answers.
PSEB 10th Class Science Important Questions Chapter 10 ਪ੍ਰਕਾਸ਼-ਪਰਾਵਰਤਨ ਅਤੇ ਅਪਵਰਤਨ
ਵੱਡੇ ਉੱਚਰਾਂ ਵਾਲੇ ਪ੍ਰਸ਼ਨ (Long Answer Type Questions)
ਪ੍ਰਸ਼ਨ 1.
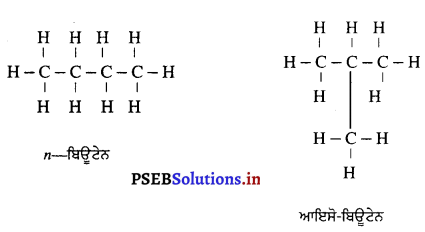
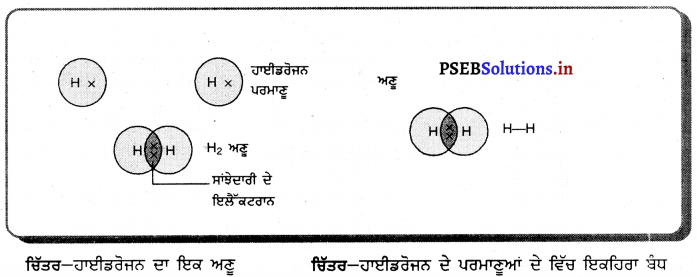
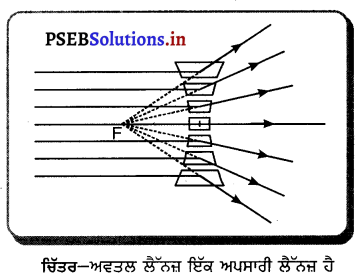
ਅਵਤਲ ਦਰਪਣ ਦੇ ਸਾਹਮਣੇ ਵਿਭਿੰਨ ਸਥਿਤੀਆਂ ‘ਤੇ ਰੱਖੀ ਵਸਤੂ ਦੇ ਦਰਪਣ ਦੁਆਰਾ ਬਣੇ ਪ੍ਰਤਿਬਿੰਬਾਂ ਦੀ ਸਥਿਤੀ, ਪ੍ਰਕਿਰਤੀ ਅਤੇ ਆਕਾਰ ਚਿੱਤਰ ਦੁਆਰਾ ਸਪੱਸ਼ਟ ਕਰੋ ।
ਉੱਤਰ-
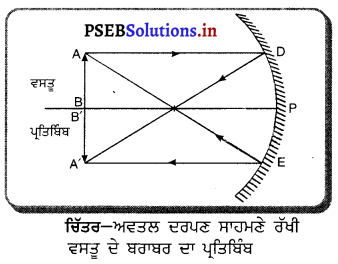
ਅਵਤਲ ਦਰਪਣ ਦੁਆਰਾ ਪ੍ਰਤਿਬਿੰਬ ਬਣਨਾ – ਅਵਤਲ ਦਰਪਣ ਦੁਆਰਾ ਬਣੇ ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਸਥਿਤੀ, ਪ੍ਰਕਿਰਤੀ ਅਤੇ ਆਕਾਰ ਦਰਪਣ ਤੋਂ ਵਸਤੂ ਦੀ ਦੂਰੀ ਤੇ ਨਿਰਭਰ ਕਰਦੀ ਹੈ ।
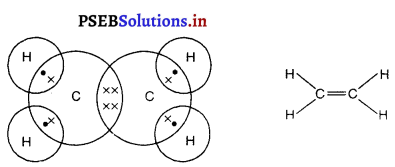
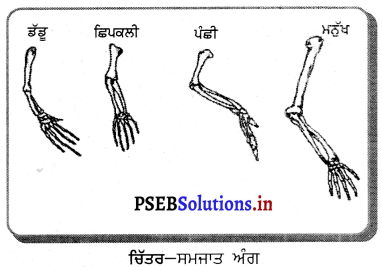
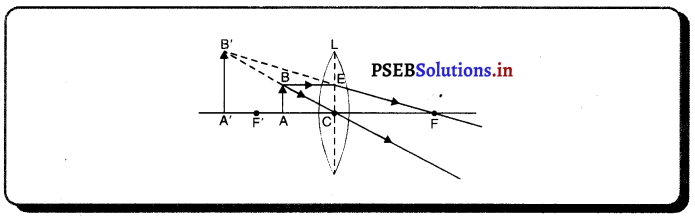
(1) ਜਦੋਂ ਵਸਤੂ ਅਨੰਤ ਦੂਰੀ ‘ਤੇ ਹੋਵੇ – ਅਨੰਤ ਦੂਰੀ ‘ਤੇ ਰੱਖੀ ਵਸਤੂ ਤੋਂ ਚਲਣ ਵਾਲੀਆਂ ਕਿਰਨਾਂ ਇੱਕ-ਦੂਜੇ ਦੇ ਸਮਾਨੰਤਰ ਹੁੰਦੀਆਂ ਹਨ । ਇੱਕ ਕਿਰਨ ਜਿਹੜੀ ਫੋਕਸ F ਤੋਂ ਹੋ ਕੇ ਜਾਂਦੀ ਹੈ ਪਰਾਵਰਤਨ ਤੋਂ ਬਾਅਦ ਮੁੱਖ ਧੁਰੇ ਦੇ ਸਮਾਨੰਤਰ ਹੋ ਜਾਂਦੀ ਹੈ ।
ਦੂਜੀ ਕਿਰਨ ਜਿਹੜੀ ਵਕ੍ਰਤਾ ਕੇਂਦਰ ਵਿਚੋਂ ਹੋ ਕੇ ਆਉਂਦੀ ਹੈ ਪਰਾਵਰਤਨ ਤੋਂ ਬਾਅਦ ਉਸੇ ਪੱਥ ਤੋਂ ਵਾਪਸ ਮੁੜ ਜਾਂਦੀ ਹੈ । ਇਹ ਦੋਵੇਂ ਪਰਵਰਤਿਤ ਕਿਰਨਾਂ ਦਰਪਣ ਦੇ ਫੋਕਸ ਤਲ ਤੇ ਬਿੰਦੂ B’ ਤੇ ਮਿਲਦੀਆਂ ਹਨ । ਇਸ ਤਰ੍ਹਾਂ B’, ਬਿੰਦੂ B ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਹੈ ਅਤੇ B’ ਤੋਂ ਮੁੱਖ ਧੁਰੇ ਤੇ ਖਿੱਚਿਆ ਗਿਆ ਲੰਬ ਧੁਰੇ ਨੂੰ A’ ਤੇ ਮਿਲਦਾ ਹੈ ਜਿਹੜਾ A ਦਾ, ਪ੍ਰਤਿਬਿੰਬ ਹੈ । ਇਸ ਤਰ੍ਹਾਂ A’B’ ਵਸਤੂ AB ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਬਣਦਾ ਹੈ ।
ਇਹ ਪ੍ਰਤਿਬਿੰਬ ਦਰਪਣ ਦੇ ਫੋਕਸ ਤੇ ਬਣਦਾ ਹੈ ਅਤੇ ਵਾਸਤਵਿਕ, ਉਲਟਾ ਅਤੇ ਵਸਤੂ ਤੋਂ ਬਹੁਤ ਛੋਟਾ ਬਣਦਾ ਹੈ ।
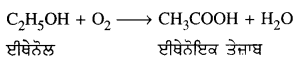
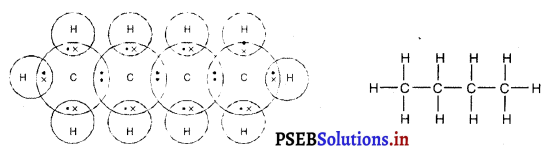
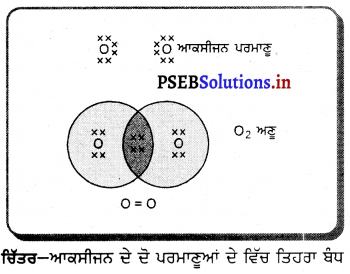
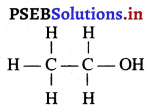
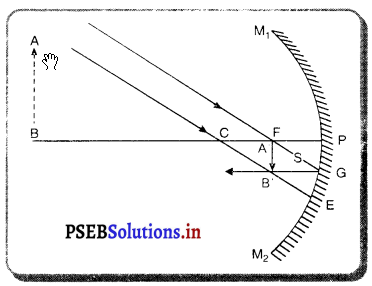
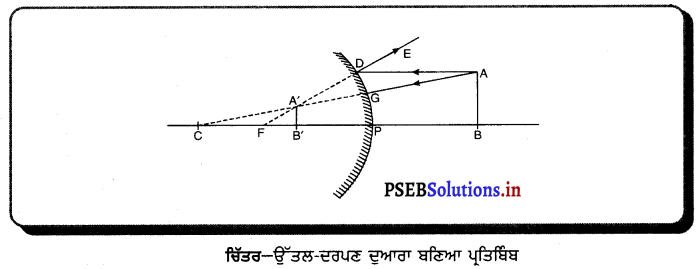
(2) ਜਦੋਂ ਵਸਤੂ ਅਨੰਤ ਅਤੇ ਵਕੁਤਾ ਕੇਂਦਰ ਦੇ ਵਿਚਕਾਰ ਰੱਖੀ ਹੋਵੇ – ਮੰਨ ਲਓ ਵਸਤੂ AB ਅਵਤਲ ਦਰਪਣ ਦੇ ਸਾਹਮਣੇ ਉਸ ਦੇ ਵਕੁਤਾ ਅਰਧ-ਵਿਆਸ ਤੋਂ ਵੱਧ ਦੁਰੀ ਤੇ ਰੱਖੀ ਗਈ ਹੈ । ਬਿੰਦੂ A ਤੋਂ ਮੁੱਖ ਧੁਰੇ ਦੇ ਸਮਾਨੰਤਰ ਚਲਣ ਵਾਲੀ ਕਿਰਨ AD ਪਰਾਵਰਤਨ ਤੋਂ ਬਾਅਦ ਫੋਕਸ ‘F ਵਿੱਚੋਂ ਹੋ ਕੇ ਲੰਘਦੀ ਹੈ । ਦੂਜੀ ਪ੍ਰਕਾਸ਼ ਕਿਰਨ AG ਜਿਹੜੀ ਵਕੁਤਾ ਕੇਂਦਰ ਵਿੱਚੋਂ ਹੋ ਕੇ ਜਾਂਦੀ ਹੈ । ਪਰਾਵਰਤਨ ਤੋਂ ਬਾਅਦ ਉਸੇ ਪੱਥ ਤੇ ਮੁੜ ਆਉਂਦੀ ਹੈ । ਇਹ ਦੋਨੋਂ ਪਰਾਵਰਤਿਤ ਕਿਰਨਾਂ A’ ਤੇ ਮਿਲਦੀਆਂ ਹਨ ਜੋ ਬਿੰਦੁ A ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਹੈ । ਬਿੰਦੁ B’ ਤੋਂ ਮੁੱਖ ਧੁਰੇ ਤੇ ਖਿੱਚਿਆ ਗਿਆ ਲੰਬ A’ ਧੁਰੇ B’ ਨੂੰ A’ ਤੇ ਮਿਲਦਾ ਹੈ, ਜੋ B ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਹੈ । ਇਸ ਤਰ੍ਹਾਂ ਵਸਤੂ AB ਦਾ ਪ੍ਰਤਿਬਿੰਬ A’B’ ਹੈ ।
ਇਹ ਤਿਬਿੰਬ ਦਰਪਣ ਦੇ ਵਕ੍ਰਤਾ ਕੇਂਦਰ C ਅਤੇ ਮੁੱਖ ਫੋਕਸ F ਦੇ ਵਿੱਚ ਬਣਦਾ ਹੈ ਜੋ ਕਿ ਵਾਸਤਵਿਕ, ਉਲਟਾ ਅਤੇ ਵਸਤੂ ਦੇ ਆਕਾਰ ਤੋਂ ਛੋਟਾ ਹੈ ।
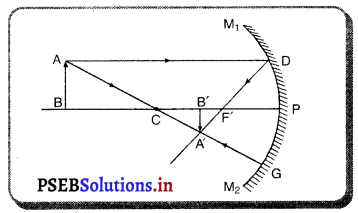
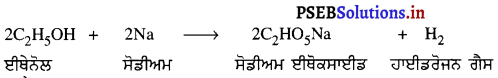
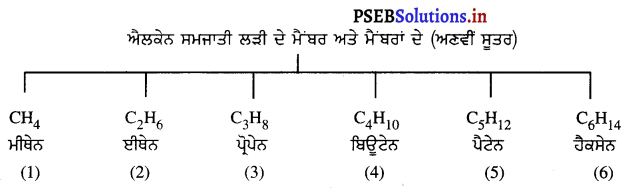
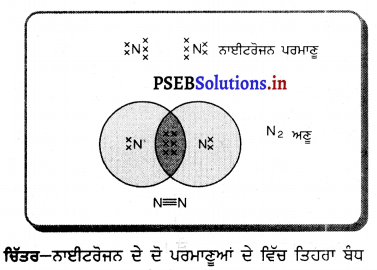
(3) ਜਦੋਂ ਵਸਤੂ ਵਕੁਤਾ ਕੇਂਦਰ ਤੇ ਰੱਖੀ ਹੋਵੇ – ਮੰਨ ਲਓ ਵਸਤੂ AB ਅਵਤਲ ਦਰਪਣ ਦੇ ਵਕੁਤਾ ਕੇਂਦਰ cਤੇ ਰੱਖੀ ਹੋਈ ਹੈ । ਬਿੰਦੁ ਨ ਤੋਂ ਮੁੱਖ ਧੁਰੇ ਦੇ ਸਮਾਨੰਤਰ ਚਲਣ ਵਾਲੀ ਆਪਾਤੀ ਕਿਰਨ AD ਪਰਾਵਰਤਨ ਹੋਣ ਤੋਂ ਬਾਅਦ ਫੋਕਸ Fਵਿੱਚੋਂ ਹੋ ਕੇ ਲੰਘਦੀ ਹੈ । ਦੂਜੀ ਆਪਾਤੀ ਕਿਰਨ AD’ ਫੋਕਸ ਵਿੱਚੋਂ ਹੋ ਕੇ ਲੰਘਦੀ ਹੈ ਜਿਹੜੀ ਪਰਾਵਰਤਨ ਹੋਣ ਤੋਂ ਬਾਅਦ ਮੁੱਖ ਧੁਰੇ ਦੇ ਸਮਾਨੰਤਰ ਹੋ ਜਾਂਦੀ ਹੈ । ਇਹ ਦੋਨੋਂ ਪਰਾਵਰਤਿਤ ਕਿਰਨਾਂ ਬਿੰਦੂ ‘ ਤੇ ਮਿਲਦੀਆਂ ਹਨ ਜੋ ਬਿੰਦੂ B ਦਾ ਵਾਸਤਵਿਕ ਪ੍ਰਤਿਬਿੰਬ ਹੈ । ਬਿੰਦੂ A’ ਤੋਂ ਖਿੱਚਿਆ ਗਿਆ ਲੰਬ ਮੁੱਖ ਧੁਰੇ ਨੂੰ B’ ਤੇ ਮਿਲਦਾ ਹੈ ਜੋ B ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਹੈ । ਇਸ ਤਰ੍ਹਾਂ A’B’ ਵਸਤੂ AB ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਬਣਦਾ ਹੈ ।
ਇਹ ਪ੍ਰਤਿਬਿੰਬ ਦਰਪਣ ਦੇ ਵਕ੍ਰਤਾ ਕੇਂਦਰ ਤੇ ਵਸਤੂ ਦੇ ਆਕਾਰ ਦੇ ਬਰਾਬਰ, ਵਾਸਤਵਿਕ ਅਤੇ ਉਲਟਾ ਹੁੰਦਾ ਹੈ ।
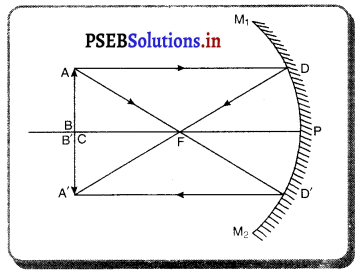
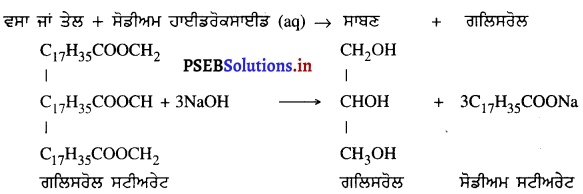
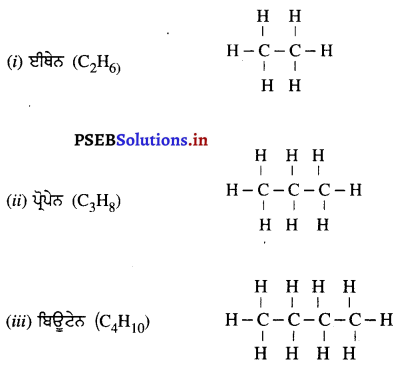
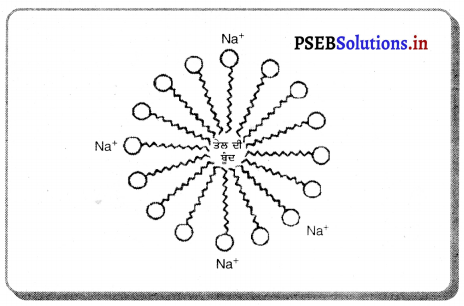
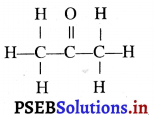
(4) ਜਦੋਂ ਵਸਤੂ ਵਕ੍ਰਤਾ ਕੇਂਦਰ Cਅਤੇ ਫੋਕਸ F ਦੇ ਵਿੱਚ ਰੱਖੀ ਹੋਵੇ – ਮੰਨ ਲਓ ਵਸਤੂ AB ਅਵਤਲ ਦਰਪਣ ਦੇ ਮੁੱਖ ਫੋਕਸ ਅਤੇ ਵੜਤਾ ਕੇਂਦਰ ਦੇ ਵਿੱਚ ਸਥਿਤ ਹੈ । ਬਿੰਦੁ A ਤੋਂ ਮੁੱਖ ਧੁਰੇ ਦੇ ਸਮਾਨੰਤਰ ਚਲਣ ਵਾਲੀ ਕਿਰਨ AD ਪਰਾਵਰਤਿਤ ਹੋ ਕੇ ਮੁੱਖ ਫੋਕਸ ਵਿੱਚੋਂ ਗੁਜਰਦੀ ਹੈ । ਦੂਜੀ ਅਯਾਤੀ ਕਿਰਨ AD’ ਮੁੱਖ ਫੋਕਸ F ਵਿਚੋਂ ਹੋ ਕੇ ਆਉਂਦੀ ਹੈ ਤੇ ਪਰਾਵਰਤਨ ਤੋਂ ਬਾਅਦ ਮੁੱਖ ਧੁਰੇ ਦੇ ਸਮਾਨੰਤਰ ਹੋ ਜਾਂਦੀ ਹੈ । ਇਹ ਦੋਨੋਂ ਪਰਾਵਰਤਿਤ ਕਿਰਨਾਂ ਇੱਕ-ਦੂਜੇ ਨੂੰ । A’ ਤੇ ਕੱਟਦੀਆਂ ਹਨ । ਜੋ ਬਿੰਦੂ A’ ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਹੈ । A’ ਤੋਂ ਮੁੱਖ ਧੁਰੇ ਤੇ ਖਿੱਚਿਆ ਗਿਆ ਲੰਬ A’B’ ਧੁਰੇ ਨੂੰ B’ ਤੇ ਮਿਲਦਾ ਹੈ ਜੋ A ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਹੈ ਇਸ ਤਰ੍ਹਾਂ ਵਸਤੂ AB ਦਾ ਪ੍ਰਤੀਬਿੰਬ A’B’ ਬਣਦਾ ਹੈ ।
ਇਹ ਤਿਬਿੰਬ ਦਰਪਣ ਦੇ ਵਤਾ ਕੇਂਦਰ ਅਤੇ ਅਨੰਤ ਦੇ ਵਿੱਚ ਬਣਦਾ ਹੈ ਅਤੇ ਵਾਸਤਵਿਕ, ਉਲਟਾ ਅਤੇ ਵਸਤੂ ਤੋਂ ਆਕਾਰ ਵਿੱਚ ਵੱਡਾ ਹੁੰਦਾ ਹੈ ।
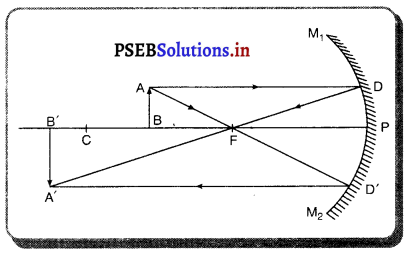
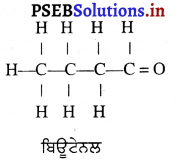
(5) ਜਦੋਂ ਵਸਤੂ ਮੁੱਖ ਫੋਕਸ ‘ਤੇ ਰੱਖੀ ਹੋਵੇ – ਮੰਨ ਲਓ ਵਸਤੂ AB ਅਵਤਲ ਦਰਪਣ ਦੇ ਮੁੱਖ ਫੋਕਸ Fਤੇ ਸਥਿਤ ਹੈ । ਇਸਦੇ ਬਿੰਦੂ A ਤੋਂ ਮੁੱਖ ਧੁਰੇ ਦੇ ਸਮਾਨੰਤਰ ਚਲਣ ਵਾਲੀ ਆਪਾਤੀ ਕਿਰਨ AD ਪਰਾਵਰਤਿਤ ਹੋ ਕੇ ਮੁੱਖ ਫੋਕਸ ਵਿੱਚੋਂ ਲੰਘਦੀ ਹੈ । ਬਿੰਦੂ A ਤੋਂ ਨਿਕਲਣ ਵਾਲੀ ਇੱਕ ਹੋਰ ਆਪਾਤੀ ਕਿਰਨ AE ਪਿੱਛੇ ਵਧਾਉਣ ਤੇ ਵਕੁਤਾ ਕੇਂਦਰ ਤੋਂ ਲੰਘਦੀ ਹੋਈ ਪ੍ਰਤੀਤ ਹੁੰਦੀ ਹੈ। ਜਿਹੜੀ ਦਰਪਣ ਤੋਂ ਪਰਾਵਰਤਿਤ ਹੋ ਕੇ ਉਸੇ ਪੱਥ ‘ਤੇ ਮੁੜ ਜਾਂਦੀ ਹੈ । ਇਹ ਦੋਨੋਂ ਪਰਾਵਰਤਿਤ ਕਿਰਨਾਂ ਇੱਕਦੂਜੇ ਦੇ ਸਮਾਨੰਤਰ ਹੋਣ ਕਾਰਨ ਅਨੰਤ ਤੇ ਮਿਲਦੀਆਂ ਹਨ ਜਿੱਥੇ ਪ੍ਰਤਿਬਿੰਬ ਬਣਦਾ ਹੈ ।
ਇਹ ਪ੍ਰਤਿਬਿੰਬ ਅਨੰਤ ਤੇ ਬਣਦਾ ਹੈ ਅਤੇ ਵਾਸਤਵਿਕ, ਉਲਟਾ ਅਤੇ ਆਕਾਰ ਵਿੱਚ ਵਸਤੂ ਤੋਂ ਵੱਡਾ ਹੁੰਦਾ ਹੈ ।
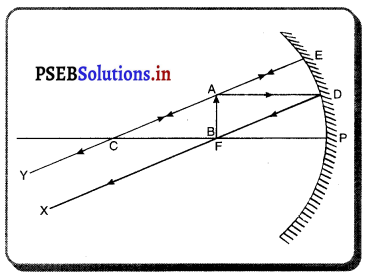
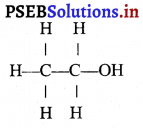
(6) ਜਦੋਂ ਵਸਤੂ ਧਰੁਵ P ਅਤੇ ਫੋਕਸ F ਦੇ ਵਿੱਚ ਰੱਖੀ ਹੈ-ਮੰਨ ਲਓ ਵਸਤੂ AB ਅਵਤਲ ਦਰਪਣ ਦੇ ਮੁੱਖ ਫੋਕਸ ਅਤੇ ਧਰੁਵ ਵਿੱਚ ਸਥਿਤ ਹੈ । ਬਿੰਦੂ A ਤੋਂ ਮੁੱਖ ਧੁਰੇ ਦੇ ਸਮਾਨੰਤਰ ਚਲਣ ਵਾਲੀ ਕਿਰਨ AD ਪਰਾਵਰਤਿਤ ਹੋ ਕੇ ਮੁੱਖ ਫੋਕਸ ਵਿੱਚੋਂ ਲੰਘਦੀ ਹੈ । ਇੱਕ-ਦੂਜੀ ਕਿਰਨ AE ਦਰਪਣ ਤੇ ਲੰਬ ਰੂਪ ਵਿੱਚ ਟਕਰਾਉਂਦੀ ਹੈ ਜਿਹੜੀ ਪਰਾਵਰਤਨ ਤੋਂ ਬਾਅਦ ਉਸੇ ਪੱਥ ‘ਤੇ ਮੁੜ ਆਉਂਦੀ ਹੈ । ਇਹ ਦੋਨੋਂ ਪਰਾਵਰਤਿਤ ਕਿਰਨਾਂ ਦਰਪਣ ਦੇ ਪਿੱਛੇ A’ ਤੋਂ ਆਉਂਦੀਆਂ ਹੋਈਆਂ ਪਤੀਤ ਹੁੰਦੀਆਂ ਹਨ । ਇਸ ਲਈ A’ ਬਿੰਦੂ A ਦਾ ਆਭਾਸੀ ਪ੍ਰਤਿਬਿੰਬ ਹੈ । ਹੁਣ A’ ਤੋਂ ਮੁੱਖ ਧੁਰੇ ਤੇ ਖਿੱਚਿਆ ਗਿਆ ਲੰਬ ਧੁਰੇ ਨੂੰ B’ ਤੇ ਮਿਲਦਾ ਹੈ ਜੋ ਬਿੰਦੂ B ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਹੈ । ਇਸ ਤਰ੍ਹਾਂ ਵਸਤੂ AB ਦਾ ਪ੍ਰਤਿਬਿੰਬ A’B’ ਬਣਦਾ ਹੈ ।
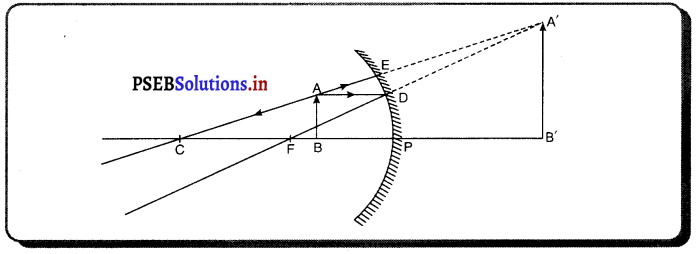
ਇਹ ਤਿਬਿੰਬ ਦਰਪਣ ਦੇ ਪਿੱਛੇ ਬਣਦਾ ਹੈ ਅਤੇ ਆਭਾਸੀ, ਸਿੱਧਾ ਅਤੇ ਆਕਾਰ ਵਿੱਚ ਵਸਤੂ ਤੋਂ ਵੱਡਾ ਹੁੰਦਾ ਹੈ ।

ਪ੍ਰਸ਼ਨ 2.
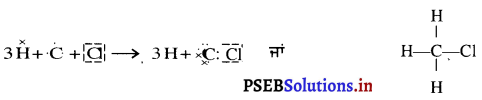
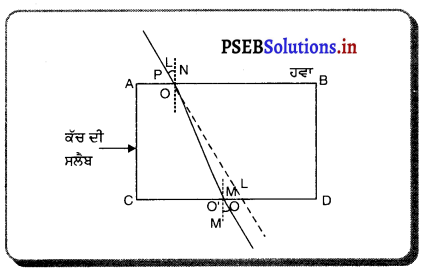
ਪ੍ਰਕਾਸ਼ ਅਪਵਰਤਨ ਕਿਸਨੂੰ ਕਹਿੰਦੇ ਹਨ ? ਕੱਚ ਦੀ ਆਇਤਾਕਾਰ ਸਲੈਬ ਵਿੱਚ ਪ੍ਰਕਾਸ਼ ਅਪਵਰਤਨ ਨੂੰ ਚਿੱਤਰ ਬਣਾ ਕੇ ਸਮਝਾਓ ਅਤੇ ਇਹ ਦਰਸਾਓ ਕਿ ਨਿਰਗਤ ਕਿਰਨ, ਆਪਾਤੀ ਕਿਰਨ ਦੇ ਸਮਾਨੰਤਰ ਹੁੰਦੀ ਹੈ ?
ਉੱਤਰ-
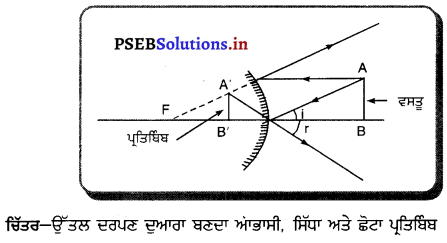
ਪ੍ਰਕਾਸ਼ ਅਪਵਰਤਨ – ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਦੀ ਕਿਰਨ ਇੱਕ ਮਾਧਿਅਮ ਤੋਂ ਦੂਜੇ ਮਾਧਿਅਮ ਵਿੱਚ ਦਾਖ਼ਲ ਹੁੰਦੀ ਹੈ, ਤਾਂ ਉਹ ਦੋ ਮਾਧਿਅਮਾਂ ਦੇ ਮਿਲਣ ਤਲ ਤੇ ਆਪਣਾ ਪੱਥ ਬਦਲ ਲੈਂਦੀ ਹੈ । ਇਸ ਪ੍ਰਕਿਰਿਆ ਨੂੰ ਪ੍ਰਕਾਸ਼ ਦਾ ਅਪਵਰਤਨ ਕਹਿੰਦੇ ਹਨ ।
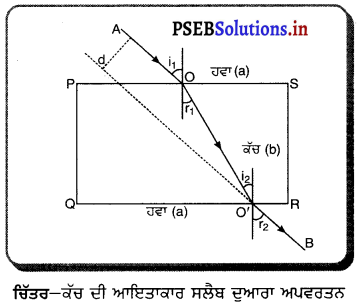
ਇਕ ਆਇਤਾਕਾਰ ਕੱਚ ਦੀ ਸਲੈਬ ਤੋਂ ਅਪਵਰਤਨ – ਇੱਕ ਆਇਤਾਕਾਰ ਕੱਚ ਦੀ ਸਲੈਬ PQRS ਨੂੰ ਹਵਾ ਵਿੱਚ ਰੱਖਿਆ ਗਿਆ ਹੈ । AO ਆਪਾਤੀ ਕਿਰਨ OO’ ਅਪਵਰਤਿਤ ਕਰਨ ਅਤੇ O’B ਨਿਰਗਤ ਕਿਰਨ ਹੈ । ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਕਿਰਨ ਹਵਾ ਤੋਂ ਕੱਚ ਵਿੱਚ ਦਾਖ਼ਲ ਹੁੰਦੀ ਹੈ ਤਾਂ ਬਿੰਦੂ ) ਤੇ ਸੁਨੇਲ ਦੇ ਨਿਯਮ ਦਾ ਪ੍ਰਯੋਗ ਕਰਨ ਤੇ
\(\frac{\sin i_{1}}{\sin r_{1}}\) = aμb …… (1)
ਹੁਣ ਪ੍ਰਕਾਸ਼ ਕਿਰਨ ਕੱਚ (ਸੰਘਣੇ ਮਾਧਿਅਮ) ਤੋਂ ਹਵਾ (ਵਿਰਲੇ ਮਾਧਿਅਮ) ਵਿੱਚ ਜਾ ਰਹੀ ਹੈ । ਬਿੰਦੂ O’ ਤੇ ਸਨੇਲ ਦੇ ਨਿਯਮ ਦਾ ਪ੍ਰਯੋਗ ਕਰਨ ਤੇ
bμa = \(\frac{\sin i_{2}}{\sin r_{2}}=\frac{\sin r_{1}}{\sin r_{2}}\) ………….. (2)
ਪ੍ਰਕਾਸ਼ ਦੇ ਉਲਟਕ੍ਰਮਣਤਾ ਨਿਯਮ (Principle of reversibility of light) ਅਨੁਸਾਰ
(∵ ∠i2 = ∠r1)
bμa = \(\frac{1}{a_{\mu_{b}}}\) ……….(3)
ਸਮੀਕਰਨ (2) ਅਤੇ (3) ਤੋਂ
aμb = \(\frac{\sin r_{2}}{\sin r_{1}}\) …………. (4)
ਸਮੀਕਰਨ (1) ਅਤੇ (4) ਤੋਂ
\(\frac{\sin i_{1}}{\sin r_{2}}=\frac{\sin r_{2}}{\sin r_{1}}\)
∴ ∠i1 = ∠r2,
ਇਸਦਾ ਅਰਥ ਹੈ ਕਿ ਆਪਨ ਕੋਣ ਨਿਰਗਤ ਕੋਣ, ਦੇ ਬਰਾਬਰ ਹੈ । ਇਸ ਲਈ ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਇੱਕ ਆਇਤਾਕਾਰ ਕੱਚ ਦੀ ਸਲੈਬ ਤੋਂ ਅਪਵਰਤਿਤ ਹੁੰਦਾ ਹੈ, ਤਾਂ ਨਿਰਗਤ ਕਰਨ ਅਤੇ ਆਪਾਤੀ ਕਿਰਨ ਸਮਾਨੰਤਰ ਹੁੰਦੀ ਹੈ ।
ਪ੍ਰਸ਼ਨ 3.
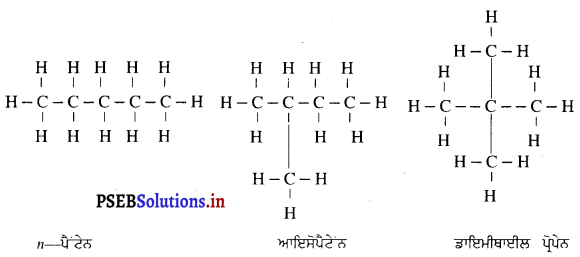
ਜਦੋਂ ਕਿਸੇ ਵਸਤੂ ਨੂੰ ਇੱਕ ਉੱਤਲ ਲੈੱਨਜ਼ ਤੋਂ (i) F ਅਤੇ 27 ਵਿਚਕਾਰ (i) 27 ਤੋਂ ਪਰੇ (ii) Fਉੱਤੇ ਰੱਖਿਆ ਜਾਂਦਾ ਹੈ ਤਾਂ ਇੱਕ ਚਿੱਤਰ ਦੀ ਸਹਾਇਤਾ ਨਾਲ ਇਸ ਦੇ ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਰਚਨਾ ਦਰਸਾਓ ।
ਜਾਂ
ਇੱਕ ਵਸਤੂ ਕਿਸੇ ਉੱਤਲ ਲੈੱਨਜ਼ ਦੇ F ਤੇ ਪਈ ਹੈ । ਚਿੱਤਰ ਦੀ ਸਹਾਇਤਾ ਨਾਲ ਉੱਤਲ ਲੈੱਨਜ਼ ਵਿਚ ਬਣੇ ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਸਥਿਤੀ, ਆਕਾਰ ਅਤੇ ਸਰੂਪ ਪਤਾ ਕਰੋ ।
ਉੱਤਰ-
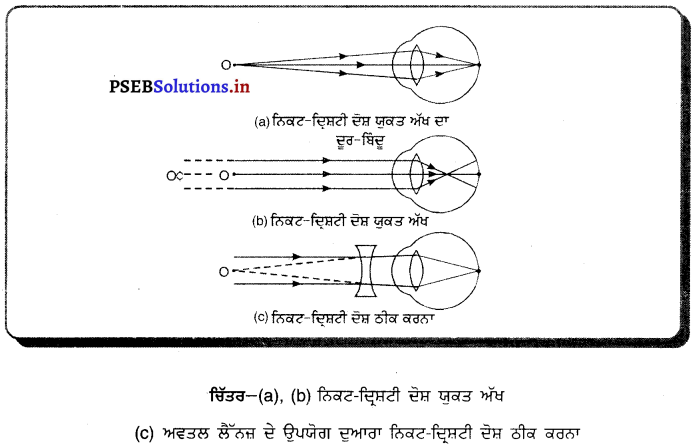
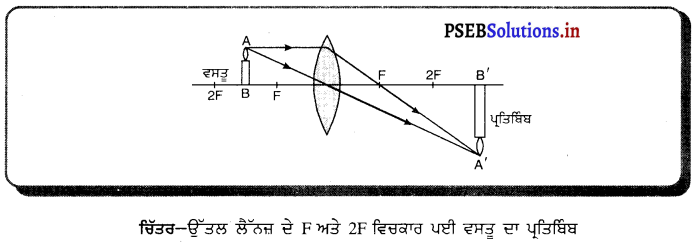
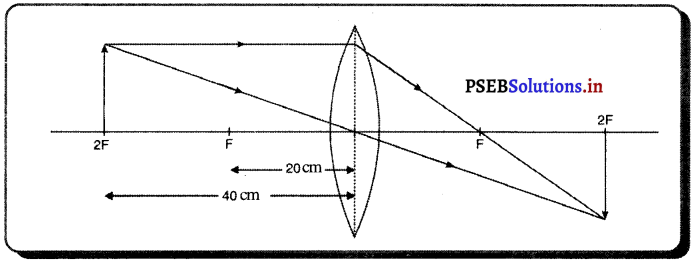
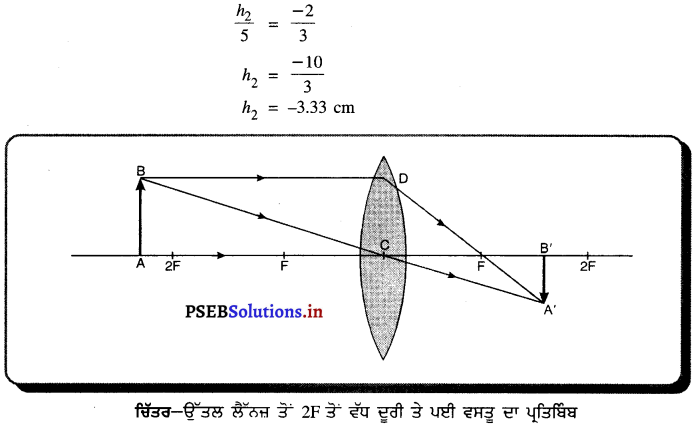
(i) ਜਦੋਂ ਵਸਤੂ F ਅਤੇ F ਦੇ ਵਿਚਕਾਰ ਹੈ – ਜਦੋਂ ਵਸਤੂ ਉੱਤਲ ਲੈੱਨਜ਼ ਦੇ F ਅਤੇ 2F ਦੇ ਵਿੱਚ ਰੱਖੀ ਜਾਂਦੀ ਹੈ ਤਾਂ ਪ੍ਰਤਿਬਿੰਬ ਲੈਂਨਜ਼ ਦੇ ਦੂਜੇ ਪਾਸੇ 2F ਤੋਂ ਪਰੇ ਬਣਦਾ ਹੈ । ਇਹ ਪ੍ਰਤਿਬਿੰਬ ਅਸਲੀ, ਉਲਟਾ ਅਤੇ ਵੱਡੇ ਸਾਇਜ਼ ਦਾ ਹੁੰਦਾ ਹੈ ।
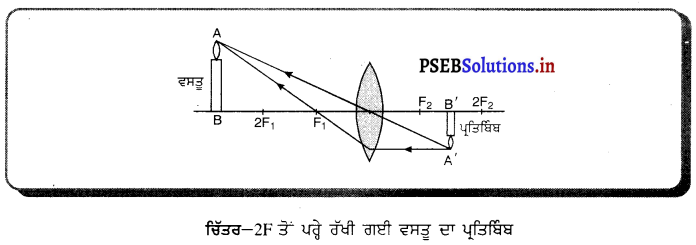
(ii) ਜਦੋਂ ਵਸਤੂ 27 ਤੋਂ ਪਰੇ ਰੱਖੀ ਹੈ – ਜਦੋਂ ਵਸਤੂ ਉੱਤਲ ਲੈੱਨਜ਼ ਦੇ ਸਾਹਮਣੇ 2F ਤੋਂ ਪਰੇ ਰੱਖੀ ਹੈ ਤਾਂ ਪ੍ਰਤਿਬਿੰਬ ਲੈਂਨਜ਼ ਦੇ ਦੂਜੇ ਪਾਸੇ Fਅਤੇ 2F ਦੇ ਵਿੱਚ ਬਣਦਾ ਹੈ । ਇਹ ਪ੍ਰਤਿਬਿੰਬ ਵਾਸਤਵਿਕ, ਉਲਟਾ ਅਤੇ ਛੋਟੇ ਸਾਇਜ਼ ਦਾ ਹੁੰਦਾ ਹੈ ।
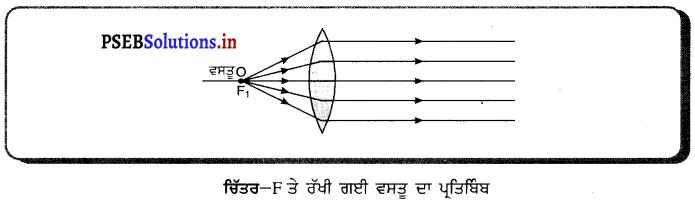
(iii) ਜਦੋਂ ਵਸਤੂ F ਤੇ ਰੱਖੀ ਹੈ – ਜਦੋਂ ਵਸਤੂ ਉੱਤਲ ਲੈੱਨਜ਼ ਦੇ F ਤੇ ਰੱਖੀ ਹੈ, ਤਾਂ ਤਿਬਿੰਬ ਲੈੱਨਜ਼ ਦੇ ਦੂਜੇ ਪਾਸੇ ਅਨੰਤ ਤੇ ਬਣਦਾ ਹੈ । ਇਹ ਪ੍ਰਤਿਬਿੰਬ ਵਾਸਤਵਿਕ ਉਲਟਾ ਅਤੇ ਸਾਇਜ਼ ਵਿੱਚ ਵੱਡਾ ਬਣਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 4.
ਚਿੱਤਰ ਵਿਚ ਕਿਹੜੀ ਕਿਰਿਆ ਦਰਸਾਈ ਗਈ ਹੈ ? ਇਸ ਦੀ ਪਰਿਭਾਸ਼ਾ ਦਿਓ ਅਤੇ ਇਸ ਦੇ ਨਿਯਮ ਵੀ ਲਿਖੋ ।
ਉੱਤਰ-
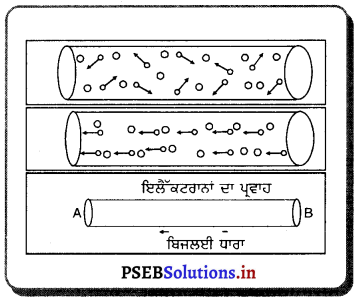
ਚਿੱਤਰ ਵਿਚ ਪ੍ਰਕਾਸ਼ ਅਪਵਰਤਨ ਦੀ ਕਿਰਿਆ ਦਰਸਾਈ ਗਈ ਹੈ ।
ਪ੍ਰਕਾਸ਼ ਦਾ ਅਪਵਰਤਨ (Refraction of Light) – ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਇੱਕ ਮਾਧਿਅਮ ਤੋਂ ਦੂਜੇ ਮਾਧਿਅਮ ਵਿੱਚ ਪ੍ਰਵੇਸ਼ ਕਰਦਾ ਹੈ ਤਾਂ ਇਹ ਆਪਣੇ ਪਹਿਲੇ ਪੱਥ ਤੋਂ ਮੁੜ ਜਾਂਦਾ ਹੈ । ਪ੍ਰਕਾਸ਼ ਦੇ ਪੱਥ ਵਿੱਚ ਹੋਈ ਇਸ ਤਬਦੀਲੀ ਨੂੰ ਪ੍ਰਕਾਸ਼ ਦਾ ਅਪਵਰਤਨ ਕਿਹਾ ਜਾਂਦਾ ਹੈ ।
ਜੇ ਪ੍ਰਕਾਸ਼ ਕਿਰਨ ਪ੍ਰਕਾਸ਼ੀ ਤੌਰ ‘ਤੇ ਵਿਰਲੇ ਮਾਧਿਅਮ ਤੋਂ ਸੰਘਣੇ ਮਾਧਿਅਮ ਵਿੱਚ ਜਾਂਦੀ ਹੈ ਤਾਂ ਉਹ ਆਪਨ ਬਿੰਦੂ ਤੇ ਬਣੇ ਅਭਿਲੰਬ ਵੱਲ ਮੁੜ ਜਾਂਦੀ ਹੈ ਅਤੇ ਜੇਕਰ ਪ੍ਰਕਾਸ਼ ਕਿਰਨ ਸੰਘਣੇ ਮਾਧਿਅਮ ਤੋਂ ਵਿਰਲੇ ਮਾਧਿਅਮ ਵਿੱਚ ਜਾਂਦੀ ਹੈ, ਤਾਂ ਇਹ ਅਭਿਲੰਬ ਤੋਂ ਪਰੇ ਮੁੜ ਜਾਂਦੀ ਹੈ ।
ਪ੍ਰਕਾਸ਼ ਅਪਵਰਤਨ ਦੇ ਨਿਯਮ – ਪ੍ਰਕਾਸ਼ ਅਪਵਰਤਨ ਦੇ ਦੋ ਨਿਯਮ ਹਨ-
(1) ਆਪਾਤੀ ਕਿਰਨ, ਅਪਵਰਤਿਤ ਕਰਨ ਅਤੇ ਅਭਿਲੰਬ ਹਮੇਸ਼ਾ ਇੱਕ ਹੀ ਤਲ ਵਿੱਚ ਹੁੰਦੇ ਹਨ ।
(2) ਜਦੋਂ ਇੱਕ ਪ੍ਰਕਾਸ਼ ਕਿਰਨ ਕਿਸੇ ਦੋ ਮਾਧਿਅਮਾਂ ਦੀ ਸੀਮਾ ਤਲ ਤੇ ਤਿਰਛੀ ਆਪਤਿਤ ਹੁੰਦੀ ਹੈ ਤਾਂ ਆਪਤਨ ਕੋਣ (∠i) ਦਾ ਸਾਈਨ (sin i) ਅਤੇ ਅਪਵਰਤਨ ਕੋਣ (∠r) ਦਾ ਸਾਈਨ (sin r) ਦਾ ਅਨੁਪਾਤ ਇੱਕ ਨਿਯਤ ਅੰਕ ਹੁੰਦਾ ਹੈ । ਇਸ ਨਿਯਤ ਅੰਕ ਨੂੰ ਦੂਜੇ ਮਾਧਿਅਮ ਦਾ ਪਹਿਲੇ ਮਾਧਿਅਮ ਦੇ ਸਾਪੇਖ ਅਪਵਰਤਨ ਅੰਕ ਕਹਿੰਦੇ ਹਨ । ਇਸਨੂੰ , ਨਾਲ ਪ੍ਰਗਟਾਇਆ ਜਾਂਦਾ ਹੈ । ਪ੍ਰਕਾਸ਼ ਦੇ ਦੂਸਰੇ ਨਿਯਮ ਨੂੰ ਸਨੌਲ ਦਾ ਨਿਯਮ ਵੀ ਕਿਹਾ ਜਾਂਦਾ ਹੈ ।

ਛੋਟੇ ਉੱਤਰਾਂ ਵਾਲੇ ਪ੍ਰਸ਼ਨ (Short Answer Type Questions)
ਪ੍ਰਸ਼ਨ 1.
ਪ੍ਰਕਾਸ਼ ਕੀ ਹੈ ? ਇਸ ਦੀ ਪ੍ਰਕਿਰਤੀ ਕੀ ਹੈ ?
ਉੱਤਰ-
ਪ੍ਰਕਾਸ਼ (Light) – ਪ੍ਰਕਾਸ਼ ਉਹ ਭੌਤਿਕ ਸਾਧਨ ਹੈ ਜੋ ਸਾਨੂੰ ਵਸਤੂਆਂ ਨੂੰ ਵੇਖਣ ਵਿੱਚ ਸਹਾਇਤਾ ਕਰਦਾ ਹੈ । ਪ੍ਰਕਾਸ਼ ਵਿਖਾਈ ਨਹੀਂ ਦਿੰਦਾ । ਇਹ ਇੱਕ ਪ੍ਰਕਾਰ ਦੀ ਊਰਜਾ ਹੈ । ਪ੍ਰਕਾਸ਼ ਬਿਜਲੀ ਚੁੰਬਕੀ ਤਰੰਗਾਂ ਹਨ ਜੋ ਹਵਾ ਜਾਂ ਨਿਰਵਾਯੂ ਵਿੱਚ ਇੱਕ ਥਾਂ ਤੋਂ ਦੂਜੇ ਥਾਂ ਤਕ ਸਿੱਧੀ ਰੇਖਾ ਵਿੱਚ ਚਲਦੀਆਂ ਹਨ ।
ਪ੍ਰਸ਼ਨ 2.
ਪ੍ਰਕਾਸ਼ ਦੀ ਪ੍ਰਕਿਰਤੀ ਦੀਆਂ ਵਿਸ਼ੇਸ਼ਤਾਈਆਂ ਲਿਖੋ ।
ਉੱਤਰ-
- ਇਨ੍ਹਾਂ ਦੇ ਸੰਚਰਣ ਲਈ ਮਾਧਿਅਮ ਦੀ ਜ਼ਰੂਰਤ ਨਹੀਂ ਹੁੰਦੀ ਹੈ ।
- ਇਹ ਬਿਜਲਈ ਤਰੰਗਾਂ ਦੇ ਰੂਪ ਵਿੱਚ ਹੁੰਦਾ ਹੈ ।
- ਇਸ ਦੀ ਚਾਲ ਮਾਧਿਅਮ ਦੀ ਪ੍ਰਕਿਰਤੀ ਤੇ ਨਿਰਭਰ ਕਰਦੀ ਹੈ ।
ਪ੍ਰਸ਼ਨ 3.
ਪ੍ਰਕਾਸ਼ ਦੇ ਬਣਾਵਟੀ ਸਰੋਤ ਕਿਹੜੇ ਹਨ ? ਉਦਾਹਰਨ ਦਿਓ ।
ਜਾਂ
ਮਨੁੱਖ ਦੁਆਰਾ ਬਣਾਏ ਗਏ ਪ੍ਰਕਾਸ਼ ਦੇ ਸੋਮੇ ਕਿਹੜੇ ਹਨ ? ਉਦਾਹਰਨ ਦਿਓ ।
ਉੱਤਰ-
ਪ੍ਰਕਾਸ਼ ਦੇ ਬਣਾਵਟੀ ਸੋਮੇ (Artificial Sources of light) – ਪ੍ਰਕਾਸ਼ ਦੇ ਮੁੱਖ ਬਣਾਵਟੀ ਸਰੋਤ ਹਨ- ਅੱਗ, ਬਿਜਲੀ, ਗੈਸ, ਦੀਪ ਅਤੇ ਕੁੱਝ ਰਸਾਇਣਿਕ ਕਿਰਿਆਵਾਂ ।
ਪ੍ਰਸ਼ਨ 4.
ਪਰਾਵਰਤਕ ਕੀ ਹੁੰਦਾ ਹੈ ?
ਉੱਤਰ-
ਪਰਾਵਰਤਕ (Reflector) – ਅਜਿਹੀ ਚੀਨੀ ਅਤੇ ਚਮਕੀਲੀ (ਪਾਲਸ਼ ਕੀਤੀ ਹੋਈ ਸਤਹਿ ਜੋ ਪ੍ਰਕਾਸ਼ ਕਿਰਨਾਂ ਨੂੰ ਉਸੇ ਮਾਧਿਅਮ ਵਿੱਚ ਵਾਪਸ ਮੋੜ ਦਿੰਦੀ ਹੈ ਜਿਸ ਵਿੱਚੋਂ ਇਹ ਕਿਰਨਾਂ ਆ ਰਹੀਆਂ ਹੁੰਦੀਆਂ ਹਨ, ਨੂੰ ਪਰਾਵਰਤਕ ਕਿਹਾ ਜਾਂਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 5.
ਪ੍ਰਕਾਸ਼ ਦੇ ਪਰਾਵਰਤਨ ਤੋਂ ਕੀ ਭਾਵ ਹੈ ? ਪ੍ਰਕਾਸ਼ ਦੇ ਪਰਾਵਰਤਨ ਦੇ ਨਿਯਮ ਲਿਖੋ ।
ਉੱਤਰ-
ਪ੍ਰਕਾਸ਼ ਪਰਾਵਰਤਨ (Reflection of light) – ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਦੀਆਂ ਕਿਰਨਾਂ ਕਿਸੇ ਸਮਤਲ ਅਤੇ ਚਮਕਦਾਰ ਸਤਹਿ ਤੇ ਟਕਰਾਉਂਦੀਆਂ ਹਨ, ਤਾਂ ਇੱਕ ਖ਼ਾਸ ਦਿਸ਼ਾ ਵਿੱਚ ਵਾਪਸ ਪਹਿਲੇ ਮਾਧਿਅਮ ਵਿੱਚ) ਮੁੜ ਜਾਂਦੀਆਂ ਹਨ । ਪ੍ਰਕਾਸ਼ ਦੀ ਇਸ ਪ੍ਰਕਿਰਿਆ ਨੂੰ ਪ੍ਰਕਾਸ਼ ਦਾ ਪਰਾਵਰਤਨ ਕਹਿੰਦੇ ਹਨ ।
ਪਰਾਵਰਤਨ ਦੇ ਨਿਯਮ (Laws of reflection) – (i) ਆਪਨ ਕੋਣ (∠i) ਅਤੇ ਪਰਾਵਰਤਨ ਕੋਣ (∠x) ਇੱਕ-ਦੂਜੇ ਦੇ ਬਰਾਬਰ ਹੁੰਦੇ ਹਨ । ਅਰਥਾਤ ∠i = ∠r
(ii) ਆਪਾਤੀ ਕਿਰਨ, ਪਰਾਵਰਤਿਤ ਕਰਨ ਅਤੇ ਆਪਨ ਬਿੰਦੂ ਤੇ ਅਭਿਲੰਬ (normal) ਸਾਰੇ ਇੱਕ ਤਲ ਵਿੱਚ ਹੁੰਦੇ ਹਨ ।
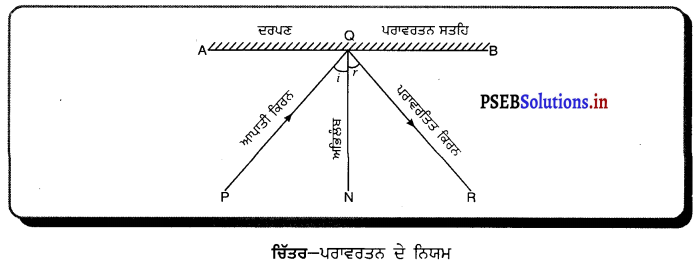
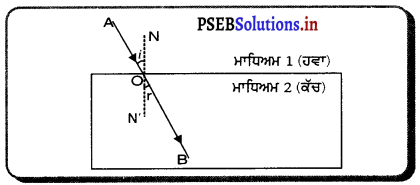
ਇਸ ਚਿੱਤਰ ਵਿੱਚ AB ਇੱਕ ਸਮਤਲ ਪਰਾਵਰਤਕ ਸਤਹਿ (ਦਰਪਣ ਹੈ, PQ ਆਪਾਤੀ ਕਿਰਨ, QR ਪਰਾਵਰਤਿਤ ਕਿਰਨ ਅਤੇ QN ਆਪਨ ਬਿੰਦੂ ਤੇ ਅਤਿਲੰਬ ਹੈ ।
ਚਿੱਤਰ ਤੋਂ ਪਤਾ ਚਲਦਾ ਹੈ ਕਿ ਆਪਾਤੀ ਕਿਰਨ, ਪਰਾਵਰਤਿਤ ਕਰਨ ਅਤੇ ਅਭਿਲੰਬ ਸਾਰੇ ਹੀ ਕਾਗ਼ਜ਼ ਦੇ ਤਲ ਵਿੱਚ ਹਨ ।
ਪ੍ਰਸ਼ਨ 6.
ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਦੀ ਕੋਈ ਕਿਰਨ ਦਰਪਣ ਤੇ ਅਭਿਲੰਬ ਰੂਪ ਵਿੱਚ ਪੈਂਦੀ ਹੈ ਤਾਂ ਆਪਤਨ-ਕੋਣ ਕਿੰਨਾ ਹੁੰਦਾ ਹੈ ?
ਉੱਤਰ-
ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਦੀ ਕੋਈ ਕਿਰਨ ਦਰਪਣ ਤੇ ਅਭਿਲੰਬ ਰੂਪ ਵਿੱਚ ਪੈਂਦੀ ਹੈ ਤਾਂ ਉਸ ਅਵਸਥਾ ਵਿੱਚ ਆਪਤਨ ਕੋਣ (∠i = 0°) ਜ਼ੀਰੋ ਡਿਗਰੀ ਦੇ ਬਰਾਬਰ ਹੁੰਦਾ ਹੈ ।

ਪ੍ਰਸ਼ਨ 7.
ਜਦੋਂ ਇੱਕ ਪ੍ਰਕਾਸ਼ ਦੀ ਕਿਰਨ ਕਿਸੇ ਦਰਪਣ ਤੇ ਲੰਬ ਰੂਪ ਵਿੱਚ ਪੈਂਦੀ ਹੈ ਤਾਂ ਇਹ ਕਿਸ ਕੋਣ ਤੇ ਪਰਾਵਰਤਿਤ ਹੁੰਦੀ ਹੈ ?
ਉੱਤਰ-
ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਦੀ ਕੋਈ ਕਿਰਨ ਕਿਸੇ ਦਰਪਣ ਤੇ ਲੰਬ ਰੂਪ ਵਿੱਚ ਪੈਂਦੀ ਹੈ, ਤਾਂ ਇਹ ਪਰਾਵਰਤਿਤ ਹੋ ਕੇ ਅਭਿਲੰਬ ਦੀ ਦਿਸ਼ਾ ਵਿੱਚ ਵਾਪਸ ਮੁੜ ਆਉਂਦੀ ਹੈ । ਇਸ ਅਵਸਥਾ ਵਿੱਚ ਪਰਾਵਰਤਨ ਕੋਣ (∠r = 0°) ਜ਼ੀਰੋ ਡਿਗਰੀ ਹੁੰਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 8.
ਕਿਸੇ ਦਰਪਣ ਤੇ ਲੰਬ ਰੂਪ ਵਿੱਚ ਪੈ ਰਹੀ ਕਿਰਨ ਮੁੜ ਉਸੇ ਰਸਤੇ ਵਾਪਸ ਆ ਜਾਂਦੀ ਹੈ । ਕਿਉਂ ?
ਉੱਤਰ-
ਕਿਸੇ ਦਰਪਣ ਤੇ ਲੰਬ ਰੂਪ ਵਿੱਚ ਪੈ ਰਹੀ ਕਿਰਨ ਉਸੇ ਰਸਤੇ ਤੇ ਵਾਪਸ ਆ ਜਾਂਦੀ ਹੈ ਕਿਉਂਕਿ ਇਸ ਅਵਸਥਾ ਵਿੱਚ ਆਪਨ ਕੋਣ (∠i = 0°) ਹੈ । ਕਿਉਂਕਿ ਪਰਾਵਰਤਨ ਦੇ ਨਿਯਮਾਂ ਅਨੁਸਾਰ ਆਪਨ ਕੋਣ ∠i = 0 ਪਰਾਵਰਤਨ ਕੋਣ ∠i ਹੁੰਦਾ ਹੈ, ਇਸ ਲਈ ਪਰਾਵਰਤਨ ਕੋਣ ∠r = 0° ਹੋਵੇਗਾ ।
ਪਸ਼ਨ 9.
ਇਨ੍ਹਾਂ ਪਦਾਂ ਦੀ ਪਰਿਭਾਸ਼ਾ ਦਿਓ-ਗੋਲਾਕਾਰ ਦਰਪਣ, ਅਵਤਲ ਦਰਪਣ, ਉੱਤਲ ਦਰਪਣ, ਦੁਆਰਕ, ਵ -ਕੇਂਦਰ, ਸ਼ੀਰਸ਼ (ਧਰੁਵ, ਮੁੱਖ ਫੋਕਸ ਅਤੇ ਫੋਕਸ-ਦੂਰੀ ।
ਉੱਤਰ-
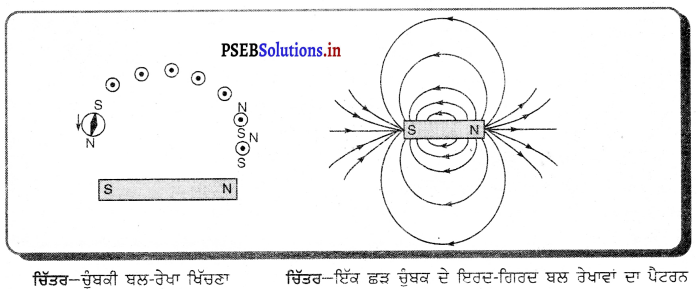
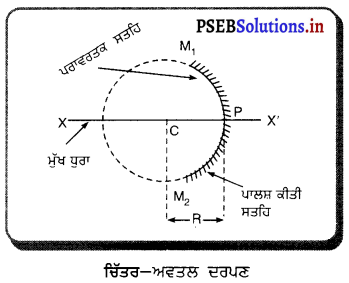
(i) ਗੋਲਾਕਾਰ ਦਰਪਣ (Spherical Mirror) – ਜੇਕਰ ਦਰਪਣ ਕਿਸੇ ਖੋਖਲੇ ਗੋਲੇ ਦਾ ਹਿੱਸਾ ਹੈ ਜਿਸ ਦੀ ਇੱਕ ਸਤਹਿ ਪਾਲਿਸ਼ ਕੀਤੀ ਹੋਈ ਅਤੇ ਦੂਜੀ ਸਤਹਿ ਪਰਾਵਰਤਕ ਹੋਵੇ ਤਾਂ ਅਜਿਹਾ ਦਰਪਣ ਗੋਲਾਕਾਰ ਦਰਪਣ ਕਹਾਉਂਦਾ ਹੈ । ਗੋਲਾਕਾਰ ਦਰਪਣ ਦੋ ਕਿਸਮ ਦੇ ਹੁੰਦੇ ਹਨ-(1) ਅਵਤਲ ਦਰਪਣ (2) ਉੱਤਲ ਦਰਪਣ ।
(ii) ਅਵਤਲ ਦਰਪਣ (Concave Mirror) – ਅਜਿਹਾ ਗੋਲਾਕਾਰ ਦਰਪਣ ਜਿਸਦੀ ਪਰਾਵਰਤਕ ਸਤਹਿ ਉਸ ਗੋਲੇ ਦੇ ਕੇਂਦਰ ਵੱਲ ਹੁੰਦੀ ਹੈ, ਜਿਸ ਦਾ ਇਹ ਦਰਪਣ ਇੱਕ ਹਿੱਸਾ ਹੈ, ਅਵਤਲ ਦਰਪਣ ਕਹਾਉਂਦਾ ਹੈ । ਅਵਤਲ ਦਰਪਣ ਦੀ ਬਾਹਰੀ ਸਤਹਿ ਪਾਲਸ਼ ਕੀਤੀ ਹੁੰਦੀ ਹੈ ਅਤੇ ਪਰਾਵਰਤਨ ਅੰਦਰਲੀ ਸਤਹਿ ਤੋਂ ਹੁੰਦਾ ਹੈ ।
(iii) ਉੱਤਲ ਦਰਪਣ (Convex Mirror) – ਅਜਿਹਾ ਦਰਪਣ ਜਿਸ ਦੀ ਪਰਾਵਰਤਕ ਸਤਹਿ ਉਸ ਗੋਲੇ ਦੇ ਕੇਂਦਰ ਤੋਂ ਪਰ੍ਹਾਂ ਵੱਲ ਹੁੰਦੀ ਹੈ ਜਿਸ ਗੋਲੇ ਦਾ ਦਰਪਣ ਹਿੱਸਾ ਹੁੰਦਾ ਹੈ, ਉੱਤਲ ਦਰਪਣ ਕਹਾਉਂਦਾ ਹੈ । ਉੱਤਲ ਦਰਪਣ ਦੀ ਅੰਦਰਲੀ ਸੜਾ ਪਾਲਿਸ਼ ਕੀਤੀ ਹੁੰਦੀ ਹੈ ਅਤੇ ਪਰਾਵਰਤਨ ਬਾਹਰੀ ਸਤਹਿ ਤੋਂ ਹੁੰਦਾ ਹੈ ।
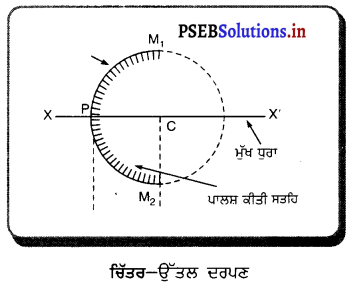
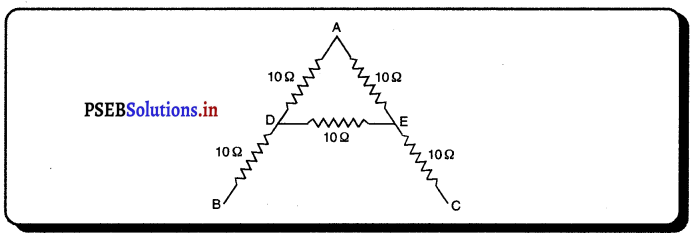
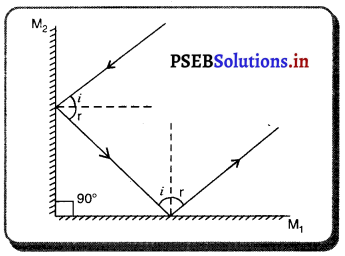
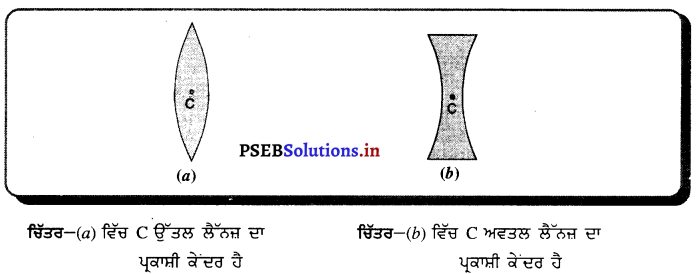
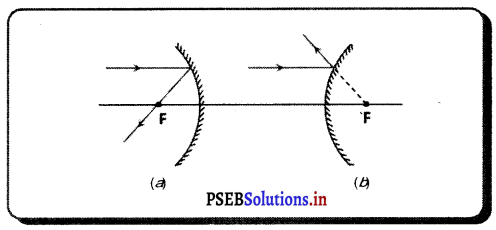
(iv) ਦੁਆਰਕ (Aperture) – ਦਰਪਣ ਦੇ ਉਸ ਹਿੱਸੇ ਨੂੰ, ਜਿਸ ਤੋਂ ਪ੍ਰਕਾਸ਼ ਅਸਲ ਵਿੱਚ ਪਰਾਵਰਤਨ ਹੁੰਦਾ ਹੈ, ਦਰਪਣ ਦਾ ਦੁਆਰਕ ਕਿਹਾ ਜਾਂਦਾ ਹੈ । ਚਿੱਤਰ (a) ਅਤੇ (b) ਵਿੱਚ ਦੂਰੀ M1M2ਦਰਪਣ ਦਾ ਦੁਆਰਕ ਹੈ ।
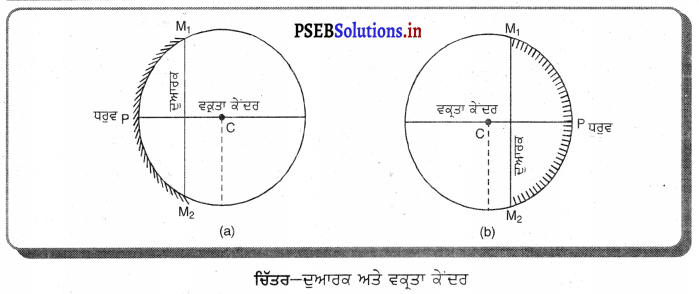
(v) ਵਾ ਕੇਂਦਰ (Centre of Curvature) – ਦਰਪਣ ਦਾ ਵਾ ਕੇਂਦਰ ਉਸ ਖੋਖਲੇ ਗੋਲੇ ਦਾ ਕੇਂਦਰ ਹੈ। ਜਿਸਦਾ ਦਰਪਣ ਇੱਕ ਹਿੱਸਾ ਹੁੰਦਾ ਹੈ । ਚਿੱਤਰ (a) ਵਿੱਚ C ਇੱਕ ਅਵਤਲ ਦਰਪਣ ਦਾ ਵਕਤਾ ਕੇਂਦਰ ਹੈ ਅਤੇ ਚਿੱਤਰ (b) ਵਿੱਚ c ਇੱਕ ਉੱਤਲ ਦਰਪਣ ਕੇਂਦਰ ਹੈ ।
(vi) ਸ਼ੀਰਸ਼ ਜਾਂ ਧਰੁਵ) (Pole) – ਕਿਸੇ ਗੋਲਾਕਾਰ ਦਰਪਣ ਦੇ ਮੱਧ ਬਿੰਦੂ ਜਾਂ ਕੇਂਦਰ ਨੂੰ ਇਸਦਾ ਧਰੁਵ ਜਾਂ ਸ਼ੀਰਸ਼ (Vertex) ਕਿਹਾ ਜਾਂਦਾ ਹੈ । ਚਿੱਤਰ (a) ਅਤੇ (b) ਵਿੱਚ ਇਸ ਨੂੰ P ਨਾਲ ਦਰਸਾਇਆ ਗਿਆ ਹੈ ।
(vii) ਮੁੱਖ ਫੋਕਸ (Principal Focus) – ਦਰਪਣ ਦਾ ਮੁੱਖ ਫੋਕਸ, ਮੁੱਖ ਧੁਰੇ ਤੇ ਉਹ ਬਿੰਦੂ ਹੁੰਦਾ ਹੈ ਜਿੱਥੇ ਸਿੱਖ ਧਰੇ ਦੇ ਸਮਾਨੰਤਰ ਆ ਰਹੀਆਂ ਪ੍ਰਕਾਸ਼ ਕਿਰਨਾਂ ਦਰਪਣ ਤੋਂ ਪਰਾਵਰਤਿਤ ਹੋ ਕੇ ਅਸਲ ਵਿੱਚ ਇੱਕ ਬਿੰਦੁ ’ਤੇ ਆ ਕੇ ਮਿਲਦੀਆਂ ਹਨ। (ਅਵਤਲ ਦਰਪਣ) ਜਾਂ ਇਸ ਬਿੰਦੂ ਤੇ ਅਭਿਸਾਰਿਤ ਹੁੰਦੀਆਂ ਹਨ ਜਾਂ ਇੱਕ ਬਿੰਦੂ ਤੋਂ ਅਪਸਰਿਤ ਹੁੰਦੀਆਂ ਜਾਪਦੀਆਂ ਹਨ (ਉੱਤਲ ਦਰਪਣ) ।
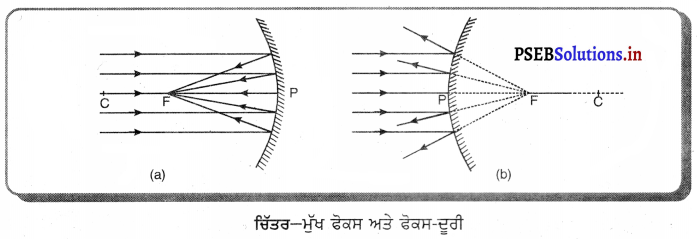
(viii) ਫੋਕਸ-ਦੁਰੀ (Focak length) – ਗੋਲਾਕਾਰ ਦਰਪਣ ਦੇ ਧਰੁਵ (pole) ਅਤੇ ਮੁੱਖ ਫੋਕਸ ਦੀ ਵਿਚਲੀ ਦੂਰੀ ਨੂੰ ਦਰਪਣ ਦੀ ਫੋਕਸ ਦੂਰੀ ਕਿਹਾ ਜਾਂਦਾ ਹੈ । ਇਸ ਨੂੰ ਨਾਲ ਦਰਸਾਇਆ ਜਾਂਦਾ ਹੈ । ਚਿੱਤਰ ਵਿੱਚ PF ਫੋਕਸ ਦੂਰੀ ਹੈ । S.I ਪੱਧਤੀ ਵਿੱਚ, ਫੋਕਸ ਦੂਰੀ ਦਾ ਮਾਤ੍ਰਿਕ ਮੀਟਰ ਹੈ ।
ਪ੍ਰਸ਼ਨ 10.
ਇੱਕ ਅਵਤਲ ਦਰਪਣ ਦੀ ਫੋਕਸ ਦੂਰੀ ਅਤੇ ਵਕੁਤਾ ਅਰਧ-ਵਿਆਸ ਵਿਚਕਾਰ ਕੀ ਸੰਬੰਧ ਹੈ ? ਸਮਤਲ ਦਰਪਣ ਦੀ ਫੋਕਸ-ਦੂਰੀ ਕਿੰਨੀ ਹੁੰਦੀ ਹੈ ?
ਉੱਤਰ-
ਇੱਕ ਅਵਤਲ ਦਰਪਣ ਦੀ ਫੋਕਸ ਦੂਰੀ ਦਰਪਣ ਦੇ ਵਕੁਤਾ ਅਰਧ-ਵਿਆਸ ਤੋਂ ਅੱਧੀ ਹੁੰਦੀ ਹੈ । ਜੇ ਅਵਲ ਦਰਪਣ ਦੀ ਫੋਕਸ ਦੂਰੀf ਅਤੇ ਵਕ੍ਰਤਾ ਅਰਧ-ਵਿਆਸ R ਹੋਵੇ, ਤਾਂ
f = \(\frac {1}{2}\) × R
ਸਮਤਲ ਦਰਪਣ ਦੀ ਫੋਕਸ ਦੂਰੀ ਅਨੰਤ ਹੁੰਦੀ ਹੈ ।

ਪ੍ਰਸ਼ਨ 11.
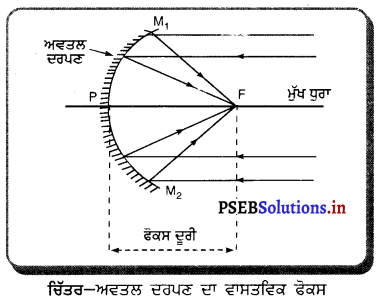
ਅਵਤਲ ਦਰਪਣ ਦਾ ਇੱਕ ਵਾਸਤਵਿਕ ਫੋਕਸ ਹੁੰਦਾ ਹੈ । ਇੱਕ ਰੇਖਾ-ਚਿੱਤਰ ਬਣਾ ਕੇ ਇਸ ਦੀ ਵਿਆਖਿਆ ਕਰੋ ।
ਉੱਤਰ-
ਅਵਤਲ ਦਰਪਣ ਦਾ ਵਾਸਤਵਿਕ ਫੋਕਸ – ਕਿਉਂਕਿ ਅਵਤਲ ਦਰਪਣ ਦੇ ਮੁੱਖ ਧੁਰੇ ਦੇ ਸਮਾਨੰਤਰ ਆ ਰਹੀਆਂ ਸਾਰੀਆਂ ਕਿਰਨਾਂ ਦਰਪਣ ਤੋਂ ਪਰਾਵਰਤਿਤ
ਮੁੱਖ ਧੁਰਾ ਹੋ ਕੇ ਅਸਲ ਵਿੱਚ ਫੋਕਸ ਵਿੱਚੋਂ ਲੰਘਦੀਆਂ ਹਨ, ਇਸ ਲਈ ਅਵਤਲ ਦਰਪਣ ਦਾ ਫੋਕਸ ਵਾਸਤਵਿਕ ਹੁੰਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 12.
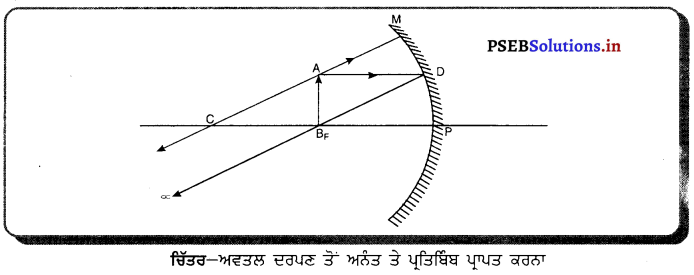
ਜਦੋਂ ਕਿਸੇ ਅਵਤਲ ਦਰਪਣ ਦੁਆਰਾ ਬਣਾਇਆ ਗਿਆ ਪ੍ਰਤਿਬਿੰਬ ਅਨੰਤ ਤੇ ਬਣਦਾ ਹੈ ਤਾਂ ਵਸਤੂ ਕਿੱਥੇ ਰੱਖੀ ਜਾਂਦੀ ਹੈ ।
ਉੱਤਰ-
ਜਦੋਂ ਵਸਤੂ ਨੂੰ ਅਵਤਲ ਦਰਪਣ ਦੇ ਫੋਕਸ ਤੇ ਰੱਖਿਆ ਜਾਂਦਾ ਹੈ ਤਾਂ ਪ੍ਰਤਿਬਿੰਬ ਅਨੰਤ ਤੇ ਬਣਦਾ ਹੈ । ਇਹ ਪਤਿਬਿੰਬ ਵਾਸਤਵਿਕ, ਉਲਟਾ ਅਤੇ ਵਸਤੂ ਦੀ ਤੁਲਨਾ ਵਿੱਚ ਵੱਡੇ ਸਾਇਜ਼ ਦਾ ਬਣਦਾ ਹੈ । ਇਸ ਅਵਸਥਾ ਵਿੱਚ ਵਸਤੁ ਤੋਂ ਆ ਰਹੀਆਂ ਪ੍ਰਕਾਸ਼ ਕਿਰਨਾਂ ਪਰਾਵਰਤਨ ਦੇ ਬਾਅਦ ਸਮਾਨੰਤਰ ਹੋ ਜਾਂਦੀਆਂ ਹਨ ।
ਪ੍ਰਸ਼ਨ 13.
ਕਿਸੇ ਵਸਤੂ ਨੂੰ ਇੱਕ ਅਵਤਲ ਦਰਪਣ ਦੇ ਸਾਹਮਣੇ ਕਿੱਥੇ ਰੱਖਿਆ ਜਾਵੇ ਤਾਂ ਜੋ ਇਸ ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਵਾਸਤਵਿਕ ਅਤੇ ਵਸਤੂ ਦੇ ਬਰਾਬਰ ਸਾਇਜ਼ ਦਾ ਬਣੇ ।
ਉੱਤਰ-
ਅਵਤਲ ਦਰਪਣ ਦੁਆਰਾ ਬਣਿਆ ਪ੍ਰਤਿਬਿੰਬ ਵਾਸਤਵਿਕ ਅਤੇ ਵਸਤੂ ਦੇ ਬਰਾਬਰ ਸਾਇਜ਼ ਦਾ ਬਣੇ, ਇਸ ਲਈ ਵਸਤੂ ਨੂੰ ਅਵਤਲ ਦਰਪਣ ਦੇ ਸਾਹਮਣੇ ਵਤਾ ਕੇਂਦਰ ਤੇ ਰੱਖਣਾ ਚਾਹੀਦਾ ਹੈ । ਇਸ ਅਵਸਥਾ ਵਿੱਚ ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਵਤਾ ਕੇਂਦਰ ਤੇ ਹੀ ਬਣਦਾ ਹੈ । ਇਹ ਤਿਬਿੰਬ ਵਾਸਤਵਿਕ ਉਲਟਾ ਅਤੇ ਸਾਇਜ਼ ਵਿੱਚ ਵਸਤੂ ਦੇ ਸਾਇਜ਼ ਦੇ ਬਰਾਬਰ ਹੁੰਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 14.
ਅਵਤਲ ਦਰਪਣ ਵਿੱਚ ਵਸਤੂ ਦਾ ਵੱਡਾ ਅਤੇ ਆਭਾਸੀ ਪ੍ਰਤਿਬਿੰਬ ਕਦੋਂ ਬਣਦਾ ਹੈ ? ਇੱਕ ਰੇਖਾ-ਚਿੱਤਰ ਦੁਆਰਾ ਦਰਸਾਓ ।
ਉੱਤਰ-
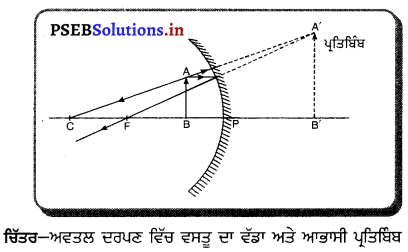
ਜਦੋਂ ਵਸਤੁ ਅਵਤਲ ਦਰਪਣ ਦੇ ਧਰੁਵ (Pole) ਅਤੇ ਫੋਕਸ ਦੇ ਵਿਚਕਾਰ ਰੱਖੀ ਜਾਂਦੀ ਹੈ, ਤਾਂ ਇਸ ਅਵਸਥਾ ਵਿੱਚ ਵਸਤੂ ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਸਿੱਧਾ, ਆਭਾਸੀ ਅਤੇ ਆਕਾਰ ਵਿੱਚ ਵਸਤੂ ਦੀ ਤੁਲਨਾ ਵਿੱਚ ਵੱਡਾ ਬਣਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 15.
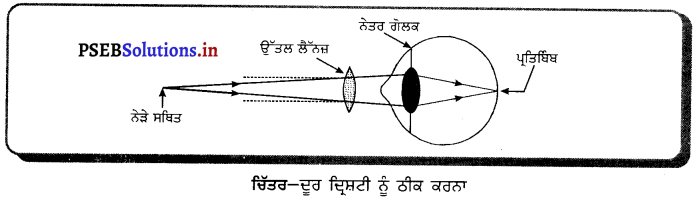
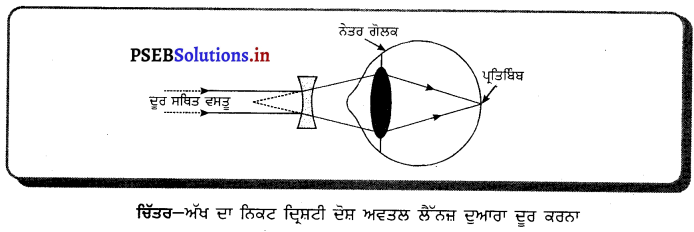
ਕਿਸ ਦਰਪਣ ਨੂੰ ਸ਼ੇਵ ਕਰਨ ਲਈ ਵਰਤਿਆ ਜਾਂਦਾ ਹੈ ਅਤੇ ਕਿਉਂ ? ਇਸ ਦੀ ਕਿਰਿਆ ਨੂੰ ਇੱਕ ਰੇਖਾਚਿੱਤਰ ਬਣਾ ਕੇ ਦਰਸਾਓ । |
ਉੱਤਰ-
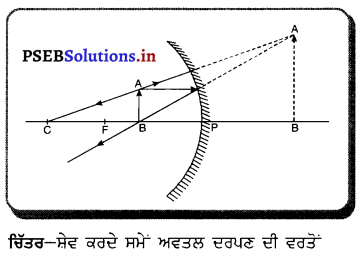
ਅਵਤਲ ਦਰਪਣ ਦਾ ਸ਼ੇਵ ਕਰਨ ਵਾਲੇ ਦਰਪਣ ਦੇ ਰੂਪ ਵਿੱਚ ਉਪਯੋਗ ਕੀਤਾ ਜਾਂਦਾ ਹੈ ਕਿਉਂਕਿ ਜਦੋਂ ਅਸੀਂ ਆਪਣਾ ਚਿਹਰਾ ਕਿਸੇ ਅਵਤਲ ਦਰਪਣ ਦੇ ਨੇੜੇ (ਧਰੁਵ ਅਤੇ ਫੋਕਸ ਦੇ ਵਿਚਕਾਰ) ਰੱਖਦੇ ਹਾਂ ਤਾਂ ਚਿਹਰੇ ਦਾ ਵੱਡਾ ਅਤੇ ਸਿੱਧਾ ਪ੍ਰਤਿਬਿੰਬ ਬਣਦਾ ਹੈ, ਜਿਸ ਨਾਲ ਬਾਰੀਕ ਵਾਲ ਵੀ ਦਿਖਾਈ ਦਿੰਦੇ ਹਨ ।
ਮਤਲਬ ਕਿ ਉਹ ਚਿਹਰੇ ਦੀ ਠੀਕ ਸ਼ੇਵ (Shave) ਕਰਨ ਵਿੱਚ ਸਹਾਈ ਹੁੰਦਾ ਹੈ । ਇਸ ਲਈ ਅਵਤਲ ਦਰਪਣ ਨੂੰ ਸ਼ੇਵ ਕਰਨ ਵਾਲੇ ਦਰਪਣ ਦੇ ਤੌਰ ਤੇ ਵਰਤਿਆ ਜਾਂਦਾ ਹੈ ।

ਪ੍ਰਸ਼ਨ 16.
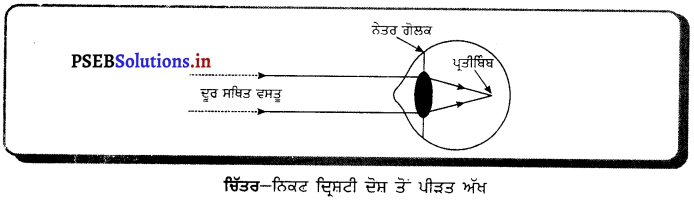
ਕਿਹੜਾ ਦਰਪਣ ਹਮੇਸ਼ਾ ਆਭਾਸੀ, ਸਿੱਧਾ ਅਤੇ ਵਸਤੂ ਨਾਲੋਂ ਛੋਟੇ ਸਾਇਜ਼ ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਬਣਾਉਂਦਾ ਹੈ ?
ਉੱਤਰ-
ਉੱਤਲ ਦਰਪਣ ਦੇ ਲਈ ਵਸਤੂ ਦੀ ਸਥਿਤੀ ਭਾਵੇਂ ਕੋਈ ਵੀ ਹੋਵੇ, ਤਿਬਿੰਬ ਹਮੇਸ਼ਾ ਆਭਾਸੀ, ਸਿੱਧਾ, ਵਸਤੂ ਤੋਂ ਛੋਟਾ ਅਤੇ ਦਰਪਣ ਦੇ ਪਿੱਛੇ ਬਣਦਾ ਹੈ । ਇਸ ਨੂੰ ਚਿੱਤਰ ਵਿੱਚ ਦਿਖਾਇਆ ਗਿਆ ਹੈ ।
ਪ੍ਰਸ਼ਨ 17.
ਕਿਸ ਦਰਪਣ ਦਾ ਦ੍ਰਿਸ਼ਟੀ-ਖੇਤਰ ਵੱਡਾ ਹੁੰਦਾ ਹੈ ?
ਉੱਤਰ-
ਉੱਤਲ ਦਰਪਣ ਵਿੱਚ ਪ੍ਰਤਿਬਿੰਬ ਹਮੇਸ਼ਾ ਆਭਾਸੀ, ਸਿੱਧਾ ਅਤੇ ਵਸਤੂ ਨਾਲੋਂ ਛੋਟੇ ਆਕਾਰ ਦਾ ਅਤੇ ਦਰਪਣ ਦੇ ਪਿੱਛੇ ਬਣਦਾ ਹੈ । ਉੱਤਲ ਦਰਪਣ ਨੂੰ ਵਸਤ ਤੋਂ ਦੂਰ ਲੈ ਜਾਣ ਤੇ, ਸਾਡੇ ਪਿੱਛੇ ਵੱਡੇ ਖੇਤਰ ਵਿੱਚ ਪਈਆਂ ਵਸਤੂਆਂ ਵੇਖੀਆਂ ਜਾ ਸਕਦੀਆਂ ਹਨ । ਇਸ ਲਈ ਉੱਤਲ ਦਰਪਣ ਦੁਆਰਾ ਪਿਛਾਂਹ ਆ ਰਹੀ ਆਵਾਜਾਈ ਦਾ ਵੱਡਾ ਖੇਤਰ ਦਿਸਦਾ ਹੈ । ਇਸ ਲਈ ਉੱਤਲ ਦਰਪਣ ਦਾ ਦ੍ਰਿਸ਼ਟੀ ਖੇਤਰ ਵੱਡਾ ਹੁੰਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 18.
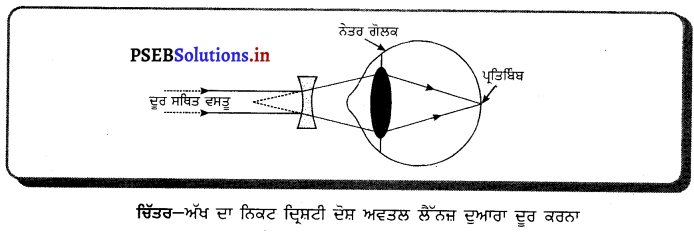
ਡਰਾਈਵਰ ਦੇ ਦਰਪਣ ਦੇ ਤੌਰ ਤੇ ਕਿਸ ਦਰਪਣ ਨੂੰ ਪਹਿਲ ਦਿੱਤੀ ਜਾਂਦੀ ਹੈ ਅਤੇ ਕਿਉਂ ? ਰੇਖਾ-ਚਿੱਤਰ ਬਣਾ ਕੇ ਦਰਸਾਓ ।
ਉੱਤਰ-
ਉੱਤਲ ਦਰਪਣ ਡਰਾਂਈਵਰ ਦੇ ਦਰਪਣ ਦੇ ਰੂਪ ਵਿੱਚ ਵਰਤਿਆ ਜਾਂਦਾ ਹੈ, ਕਿਉਂਕਿ ਉੱਤਲ ਦਰਪਣ ਵਿੱਚ ਬਣ ਰਿਹਾ ਪ੍ਰਤਿਬਿੰਬ ਵਸਤੂ ਤੋਂ ਬਹੁਤ ਛੋਟਾ ਅਤੇ ਸਿੱਧਾ ਬਣਦਾ ਹੈ । ਇਸ ਲਈ ਉੱਤਲ ਦਰਪਣ ਦੁਆਰਾ ਪਿੱਛੇ ਆ ਰਹੀ ਟਰੈਫ਼ਿਕ ਦਾ ਇੱਕ ਵੱਡਾ ਖੇਤਰ ਦਿਖਾਈ ਦਿੰਦਾ ਹੈ । ਇਸ ਲਈ ਉੱਤਲ ਦਰਪਣ ਨੂੰ ਡਰਾਈਵਰ ਦਰਪਣ ਦੇ ਰੂਪ ਵਿੱਚ ਪਹਿਲ ਦਿੱਤੀ ਜਾਂਦੀ ਹੈ ।
ਪ੍ਰਸ਼ਨ 19.
ਪਿੱਛੇ ਦੀ ਆਵਾਜਾਈ ਦੇਖਣ ਵਾਸਤੇ ਵਾਹਨਾਂ ਵਿੱਚ ਕਿਸ ਕਿਸਮ ਦੇ ਦਰਪਣ ਦੀ ਵਰਤੋਂ ਕੀਤੀ ਜਾਂਦੀ ਹੈ ?
ਉੱਤਰ-
ਕਿਉਂਕਿ ਉੱਤਲ ਦਰਪਣ ਵਿੱਚ ਪਿੱਛੇ ਆ ਰਹੀ ਟਰੈਫ਼ਿਕ ਦਾ ਸਿੱਧਾ ਅਤੇ ਛੋਟਾ ਪ੍ਰਤਿਬਿੰਬ ਬਣਦਾ ਹੈ, ਇਸ ਲਈ ਵੱਡੇ ਖੇਤਰ ਵਿੱਚ ਆ ਰਹੇ ਵਾਹਨਾਂ ਨੂੰ ਦੇਖਣ ਲਈ ਉੱਤਲ ਦਰਪਣ ਦੀ ਵਰਤੋਂ ਵਾਹਨ ਦਰਪਣ ਦੇ ਰੂਪ ਵਿੱਚ ਕੀਤਾ ਜਾਂਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 20.
ਸਮਤਲ ਦਰਪਣ, ਅਵਤਲ ਦਰਪਣ ਅਤੇ ਉੱਤਲ ਦਰਪਣ ਨੂੰ ਛੋਹੇ ਬਿਨਾਂ ਇਨ੍ਹਾਂ ਵਿੱਚ ਫ਼ਰਕ ਕਿਵੇਂ ਪਤਾ ਕਰੋਗੇ ?
ਉੱਤਰ-
ਸਾਰੇ ਦਰਪਣਾਂ ਵਿੱਚ ਆਪਣਾ ਚਿਹਰਾ ਦੇਖੋ 1 ਹੁਣ ਆਪਣਾ ਚਿਹਰਾ ਦਰਪਣ ਤੋਂ ਦੂਰ ਲਿਜਾਓ ਅਤੇ ਪ੍ਰਤਿਬਿੰਬ ਦਾ ਸਾਇਜ਼ ਨੋਟ ਕਰੋ । ਤੁਸੀਂ ਵੇਖੋਗੇ ਕਿ ਸਮਤਲ ਦਰਪਣ ਵਿੱਚ ਬਣ ਰਹੇ ਪ੍ਰਤਿਬਿੰਬ ਦਾ ਸਾਈਜ਼ ਚਿਹਰੇ ਦੇ ਸਾਈਜ਼ ਦੇ ਬਰਾਬਰ ਅਤੇ ਉੱਤਲ ਦਰਪਣ ਵਿੱਚ ਛੋਟਾ ਅਤੇ ਅਵਤਲ ਦਰਪਣ ਵਿੱਚ ਵੱਡਾ ਹੋਵੇਗਾ । ਇਸ ਤਰ੍ਹਾਂ ਅਸੀਂ ਬਿਨਾਂ ਛੋਹੇ ਸਮਤਲ, ਉੱਤਲ ਅਤੇ ਅਵਤਲ ਦਰਪਣ ਦੀ ਪਛਾਣ ਕਰ ਲਵਾਂਗੇ ।
ਪ੍ਰਸ਼ਨ 21.
ਇੱਕ ਗੋਲਾਕਾਰ ਦਰਪਣ ਦੇ ਵੱਡਦਰਸ਼ਨ ਦੀ ਪਰਿਭਾਸ਼ਾ ਦਿਓ । ਸਮਤਲ ਦਰਪਣ ਵਿੱਚ ਵੱਡਦਰਸ਼ਨ ਕਿੰਨਾ ਹੁੰਦਾ ਹੈ ?
ਉੱਤਰ-
ਵੱਡਦਰਸ਼ਨ (Magnification) – ਗੋਲਾਕਾਰ ਦਰਪਣ ਦਾ ਵੱਡਦਰਸ਼ਨ ਦਰਪਣ ਦੁਆਰਾ ਬਣਾਏ ਗਏ ਤਿਬਿੰਬ ਦੇ ਆਕਾਰ ਜਾਂ ਸਾਈਜ਼ (size) ਅਤੇ ਵਸਤੂ ਦੇ ਆਕਾਰ ਜਾਂ ਸਾਈਜ਼ (size) ਦੇ ਅਨੁਪਾਤ ਦੇ ਬਰਾਬਰ ਹੁੰਦਾ ਹੈ । ਇਸ ਨੂੰ ਅ ਨਾਲ ਪ੍ਰਦਰਸ਼ਿਤ ਕੀਤਾ ਜਾਂਦਾ ਹੈ ।

ਸਮਤਲ ਦਰਪਣ ਦਾ ਵੱਡਦਰਸ਼ਨ – ਸਮਤਲ ਦਰਪਣ ਨੂੰ ਇੱਕ ਅਜਿਹੇ ਗੋਲੇ ਦਾ ਹਿੱਸਾ ਮੰਨਿਆ ਜਾ ਸਕਦਾ ਹੈ ਜਿਸਦਾ ਅਰਧ-ਵਿਆਸ ਅਨੰਤ ਹੈ ।
∴ ਦਰਪਣ ਫਾਰਮੂਲਾ ਲਗਾਉਣ ‘ਤੇ
\(\frac{1}{u}+\frac{1}{v}=\frac{1}{f}\)
\(\frac{1}{u}+\frac{1}{v}=\frac{1}{\infty}\)
\(\frac{1}{u}+\frac{1}{v}\) = 0
ਜਾਂ u + υ = 0
u = -υ
ਹੁਣ ਵੱਡਦਰਸ਼ਨ (m) = \(\frac{-v}{u}=\frac{u}{u}\) = 1
ਅਰਥਾਤ ਵਸਤੂ ਦਾ ਆਕਾਰ ਅਤੇ ਪ੍ਰਤਿਬਿੰਬ ਦਾ ਆਕਾਰ ਇੱਕ ਸਮਾਨ ਹੁੰਦਾ ਹੈ ।

ਪ੍ਰਸ਼ਨ 22.
ਇਕ ਸਮਤਲ ਦਰਪਣ ਦੁਆਰਾ ਬਣਾਏ ਗਏ ਪ੍ਰਤਿਬਿੰਬ ਦੇ ਲੱਛਣ ਦੱਸੋ ।
ਉੱਤਰ-
ਸਮਤਲ ਦਰਪਣ ਵਿੱਚ ਬਣੇ ਪ੍ਰਤਿਬਿੰਬ ਦੇ ਲੱਛਣ (ਗੁਣ)-
- ਇਹ ਆਭਾਸੀ ਹੁੰਦਾ ਹੈ, ਭਾਵ ਕਿ ਇਸ ਨੂੰ ਪਰਦੇ ਤੇ ਪ੍ਰਾਪਤ ਨਹੀਂ ਕੀਤਾ ਜਾ ਸਕਦਾ ਹੈ ।
- ਇਹ ਸਿੱਧਾ ਹੁੰਦਾ ਹੈ ।
- ਇਹ ਪਾਸੇ-ਦਾਅ ਉਲਟਿਆ ਹੁੰਦਾ ਹੈ, ਜਿਵੇਂ ਸੱਜਾ ਹੱਥ ਦਰਪਣ ਵਿੱਚ ਖੱਬਾ ਨਜ਼ਰ ਆਉਂਦਾ ਹੈ ਅਤੇ ਖੱਬਾ ਹੱਥ, ਸੱਜਾ ਜਾਪਦਾ ਹੈ !
- ਇਸ ਦਾ ਆਕਾਰ (ਸਾਇਜ਼) ਵਸਤੂ ਦੇ ਆਕਾਰ (ਸਾਇਜ਼) ਦੇ ਬਰਾਬਰ ਹੁੰਦਾ ਹੈ ।
- ਤਿਬਿੰਬ ਦਰਪਣ ਦੇ ਪਿੱਛੇ ਓਨੀ ਦੂਰ ਹੀ ਬਣਦਾ ਹੈ ਜਿੰਨੀ ਦੂਰ ਵਸਤੂ ਦਰਪਣ ਦੇ ਸਾਹਮਣੇ ਰੱਖੀ ਜਾਂਦੀ ਹੈ ।
ਪ੍ਰਸ਼ਨ 23.
ਪ੍ਰਤਿਬਿੰਬ ਤੋਂ ਤੁਹਾਡਾ ਕੀ ਭਾਵ ਹੈ ? ਆਭਾਸੀ ਅਤੇ ਵਾਸਤਵਿਕ ਪ੍ਰਤਿਬਿੰਬ ਵਿੱਚ ਕੀ ਅੰਤਰ ਹੈ ?
ਉੱਤਰ-
ਪ੍ਰਤਿਬਿੰਥ – ਦਰਪਣ ਦੇ ਸਾਹਮਣੇ ਰੱਖੀ ਵਸਤੂ ਦੀ ਦਰਪਣ ਵਿੱਚ ਜਿਹੜੀ ਆਕਿਰਤੀ (ਸ਼ਕਲ ਬਣ ਜਾਂਦੀ ਹੈ । ਉਸ ਆਕਿਰਤੀ ਨੂੰ ਵਸਤੂ ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਕਹਿੰਦੇ ਹਨ ।
ਪਰਿਭਾਸ਼ਾ – ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਦੀਆਂ ਕਿਰਨਾਂ ਕਿਸੇ ਵਸਤੂ (ਬਿੰਦੂ) ਤੋਂ ਪਰਾਵਰਤਨ (ਦਰਪਣ ਵਿੱਚ) ਜਾਂ ਅਪਵਰਤਨ ਲੈਨਜ਼ ਵਿੱਚ) ਤੋਂ ਬਾਅਦ ਕਿਸੇ ਬਿੰਦੁ ਤੇ ਜਾ ਕੇ ਮਿਲ ਜਾਂਦੀਆਂ ਹਨ । ਜਾਂ ਫਿਰ ਕਿਸੇ ਹੋਰ ਬਿੰਦੂ ਤੋਂ ਆ ਰਹੀਆਂ ਜਾਪਦੀਆਂ ਹਨ ਤਾਂ ਇਸ ਦੂਜੇ ਬਿੰਦੂ ਨੂੰ ਪਹਿਲੇ ਬਿੰਦੂ ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਕਹਿੰਦੇ ਹਨ ।
ਵਾਸਤਵਿਕ ਪ੍ਰਤਿਬਿੰਬ ਅਤੇ ਆਭਾਸੀ ਤਿਬਿੰਬ ਵਿਚ ਅੰਤਰ-
| ਵਾਸਤਵਿਕ ਪ੍ਰਤਿਬਿੰਬ |
ਆਭਾਸੀ ਤਿਬਿੰਬ |
| (1) ਪਰਾਵਰਤਨ ਜਾਂ ਅਪਵਰਤਨ ਤੋਂ ਬਾਅਦ ਪ੍ਰਕਾਸ਼ ਦੀਆਂ ਕਿਰਨਾਂ ਆਪਸ ਵਿੱਚ ਇੱਕ-ਦੂਜੇ ਨੂੰ ਮਿਲਦੀਆਂ ਹਨ ਅਤੇ ਪ੍ਰਤਿਬਿੰਬ ਵਾਸਤਵਿਕ ਬਣਦਾ ਹੈ । |
(1) ਪਰਾਵਰਤਨ ਜਾਂ ਅਪਵਰਤਨ ਦੇ ਬਾਅਦ ਪ੍ਰਕਾਸ਼ ਦੀਆਂ ਕਿਰਨਾਂ ਆਪਸ ਵਿੱਚ ਨਹੀਂ ਮਿਲਦੀਆਂ (ਪਿੱਛੇ ਵਧਾ ਕੇ ਉਨ੍ਹਾਂ ਨੂੰ ਮਿਲਾਉਣਾ ਪੈਂਦਾ ਹੈ) ਅਤੇ ਪ੍ਰਤਿਬਿੰਬ ਆਭਾਸੀ ਬਣਦਾ ਹੈ । |
| (2) ਇਹ ਤਿਬਿੰਬ ਪਰਦੇ ਤੇ ਲਿਆਇਆ ਜਾ ਸਕਦਾ ਹੈ । |
(2) ਇਹ ਤਿਬਿੰਬ ਪਰਦੇ ਤੇ ਨਹੀਂ ਲਿਆਇਆ ਜਾ ਸਕਦਾ । |
| (3) ਇਹ ਤਿਬਿੰਬ ਹਮੇਸ਼ਾ ਉਲਟਾ ਬਣਦਾ ਹੈ । |
(3) ਇਹ ਤਿਬਿੰਬ ਹਮੇਸ਼ਾ ਸਿੱਧਾ ਬਣਦਾ ਹੈ । |

ਪ੍ਰਸ਼ਨ 24.
ਸਮਤਲ ਦਰਪਣ ਦੁਆਰਾ ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਰਚਨਾ ਚਿੱਤਰ ਸਹਿਤ ਸਮਝਾਓ ।
ਜਾਂ
ਕਿਵੇਂ ਦਰਸਾਓਗੇ ਕਿ ਸਮਤਲ ਦਰਪਣ ਵਿੱਚ ਬਣੇ ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਦਰਪਣ ਤੋਂ ਦੂਰੀ ਉਸ ਦੇ ਸਾਹਮਣੇ ਪਈ ਵਸਤੂ ਦੀ ਦਰਪਣ ਵਿੱਚ ਦੂਰੀ ਦੇ ਬਰਾਬਰ ਹੁੰਦੀ ਹੈ ?
ਉੱਤਰ-
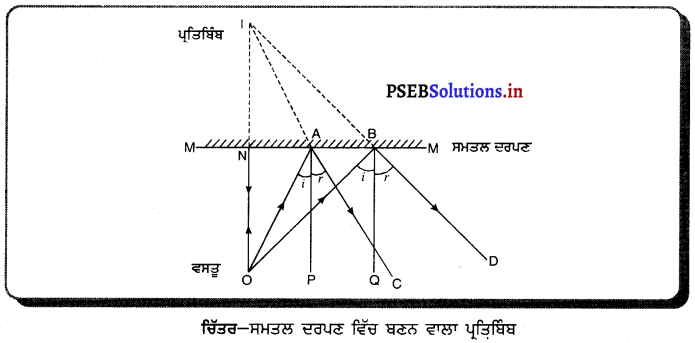
ਸਮਤਲ ਦਰਪਣ ਦੁਆਰਾ ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਰਚਨਾ-
ਮੰਨ ਲਓ MM’ ਇੱਕ ਸਮਤਲ ਦਰਪਣ ਹੈ ਅਤੇ ਉਸਦੇ ਸਾਹਮਣੇ ਵਸਤੂ O ਪਈ ਹੈ । OA ਅਤੇ OB ਦੋ-ਆਪਾਤੀ ਕਿਰਨਾਂ ਹਨ ਅਤੇ AC ਅਤੇ BD ਇਨ੍ਹਾਂ ਦੀਆਂ ਪਰਾਵਰਤਿਤ ਕਿਰਨਾਂ ਹਨ I ਪ੍ਰਤਿਬਿੰਬ ਹੈ ਕਿਉਂਕਿ ਪਰਾਵਰਤਿਤ ਕਿਰਨਾਂ ਅਸਲ ਵਿੱਚ I ਤੋਂ ਨਹੀਂ ਮਿਲਦੀਆਂ ਪਰੰਤੂ I ਤੇ ਮਿਲਦੀਆਂ ਹੋਈਆਂ ਪ੍ਰਤੀਤ ਹੁੰਦੀਆਂ ਹਨ । ਇਸ ਲਈ 1 ਵਸਤੂ ) ਦਾ ਆਭਾਸੀ ਪ੍ਰਤਿਬਿੰਬ ਹੈ ।
ਮਾਪਣ ਤੋਂ ਪਤਾ ਲਗਦਾ ਹੈ ਕਿ NO = NI, ਅਰਥਾਤ ਪ੍ਰਤਿਬਿੰਬ ਦਰਪਣ ਦੇ ਪਿੱਛੇ ਓਨੀ ਦੂਰੀ ਤੇ ਬਣਦਾ ਹੈ ਜਿੰਨੀ ਦੁਰੀ ਤੇ ਵਸਤੁ ਦਰਪਣ ਦੇ ਸਾਹਮਣੇ ਰੱਖੀ ਹੁੰਦੀ ਹੈ ।
ਪ੍ਰਸ਼ਨ 25.
ਗੋਲਾਕਾਰ ਦਰਪਣ ਦੇ ਮੁੱਖ ਉਪਯੋਗ ਲਿਖੋ ।
ਉੱਤਰ-
ਗੋਲਾਕਾਰ ਦਰਪਣ ਦੇ ਮੁੱਖ ਉਪਯੋਗਗੋਲਾਕਾਰ ਦਰਪਣ ਦੋ ਕਿਸਮ ਦੇ ਹੁੰਦੇ ਹਨ-
(1) ਅਵਤਲ ਦਰਪਣ ਅਤੇ
(2) ਉੱਤਲ ਦਰਪਣ ।
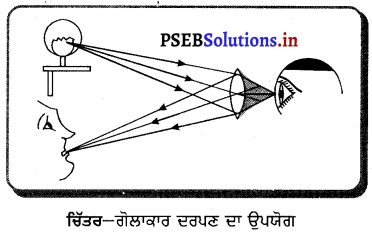
1. ਅਵਤਲ ਦਰਪਣ ਦੇ ਉਪਯੋਗ-
- ਅਵਤਲ ਦਰਪਣ, ਪਰਾਵਰਤਕ ਦੇ ਰੂਪ ਵਿੱਚ ਪ੍ਰਯੋਗ ਵਿੱਚ ਲਿਆਇਆ ਜਾਂਦਾ ਹੈ । ਵੱਡੇ ਵਿਆਸ ਵਾਲੇ ਦਰਪਣ ਨੂੰ ਪਰਾਵਰਤਕ ਦੂਰਦਰਸ਼ੀ ਵਿੱਚ ਵਰਤਿਆ ਜਾਂਦਾ ਹੈ ।
- ਇੱਕ ਅਵਤਲ ਦਰਪਣ ਜਿਸਦੇ ਕੇਂਦਰ ਵਿੱਚ ਛੇਕ ਹੋਵੇ, ਡਾਕਟਰ ਦੇ ਸਿਰ ਦੇ ਦਰਪਣ (Head Mirror) ਦੇ ਰੂਪ ਵਿੱਚ ਅੱਖ, ਨੱਕ, ਗਲੇ ਅਤੇ ਕੰਨ ਦੇ ਨਿਰੀਖਣ ਲਈ ਪ੍ਰਯੋਗ ਵਿੱਚ ਲਿਆਇਆ ਜਾਂਦਾ ਹੈ ।
- ਜਦੋਂ ਵਸਤੂ ਨੂੰ ਦਰਪਣ ਦੇ ਸ਼ੀਰਸ਼ ਅਤੇ ਫੋਕਸ ਦੇ ਵਿਚਕਾਰ ਰੱਖਿਆ ਜਾਂਦਾ ਹੈ ਤਾਂ ਇਹ ਸਿੱਧਾ, ਵੱਡਾ ਅਤੇ ਆਭਾਸੀ ਪ੍ਰਤਿਬਿੰਬ ਬਣਾਉਂਦਾ ਹੈ । ਇਸ ਲਈ ਇਸ ਨੂੰ ਸ਼ੇਵ ਦਰਪਣ ਦੇ ਰੂਪ ਵਜੋਂ ਪ੍ਰਯੋਗ ਕੀਤਾ ਜਾਂਦਾ ਹੈ ।
(2) ਉੱਤਲ ਦਰਪਣ ਦੇ ਉਪਯੋਗ-
ਉੱਤਲ ਦਰਪਣ, ਡਰਾਈਵਰਾਂ ਦੁਆਰਾ ਪਿੱਛੇ ਆ ਰਹੇ ਟਰੈਫ਼ਿਕ ਦਾ ਵਧੇਰੇ ਦ੍ਰਿਸ਼ਟੀ ਖੇਤਰ ਵੇਖਣ ਲਈ ਪ੍ਰਯੋਗ ਕੀਤਾ ਜਾਂਦਾ ਹੈ, ਕਿਉਂਕਿ ਇਸ ਦਰਪਣ ਵਿੱਚ ਪ੍ਰਤਿਬਿੰਬ ਛੋਟਾ, ਸਿੱਧਾ ਅਤੇ ਆਭਾਸੀ ਹੁੰਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 26.
ਉੱਤਲ ਦਰਪਣ ਦੁਆਰਾ ਬਣਾਏ ਗਏ ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਰਚਨਾ ਚਿੱਤਰ ਦੁਆਰਾ ਸਮਝਾਓ । ,
ਉੱਤਰ-
ਉੱਤਲ ਦਰਪਣ ਦੁਆਰਾ ਬਣਾਏ ਤਿਬਿੰਬ ਦੀ ਰਚਨਾ (Formation of Image by Convex Mirror) – ਮੰਨ ਲਓ ਇੱਕ ਵਸਤੂ AB ਉੱਤਲ ਦਰਪਣ ਦੇ ਸਾਹਮਣੇ ਇਸਦੇ ਮੁੱਖ ਧੁਰੇ ਤੇ ਪਈ ਹੈ । ਇੱਕ ਕਿਰਨ AD, A ਬਿੰਦੂ ਤੋਂ ਚਲ ਕੇ ਦਰਪਣ ਤੋਂ ਪਰਾਵਰਤਨ ਦੇ ਬਾਅਦ DE ਦਿਸ਼ਾ ਵਿੱਚ ਜਾਂਦੀ ਹੈ ਜੋ ਕਿ ਮੁੱਖ ਫੋਕਸ Fਵਿੱਚੋਂ ਆਉਂਦੀ ਵਿਖਾਈ ਦਿੰਦੀ ਹੈ । ਇੱਕ ਹੋਰ ਆਪਾਤੀ ਕਿਰਨ AGC, ਵਜ੍ਹਾ ਕੇਂਦਰ c ਤੋਂ ਲੰਘਦੀ ਹੋਈ ਜਾਪਦੀ ਪਰਾਵਰਤਿਤ ਹੋ ਕੇ, ਵਾਪਸ ਮੁੜ ਜਾਂਦੀ ਹੈ ।
ਇਹ ਦੋਨੋਂ ਪਰਾਵਰਤਿਤ ਕਿਰਨਾਂ A’ ਤੇ ਮਿਲਦੀਆਂ ਹੋਈਆਂ ਵਿਖਾਈ ਦਿੰਦੀਆਂ ਹਨ ਜੋ A ਦਾ ਆਭਾਸੀ ਪ੍ਰਤਿਬਿੰਬ ਹੈ । A’ ਤੇ ਬਣਿਆ ਲੰਬ A’B’, ਵਸਤੂ AB ਦਾ ਆਭਾਸੀ ਸਿੱਧਾ ਅਤੇ ਸਾਇਜ਼ ਵਿੱਚ ਛੋਟਾ ਪ੍ਰਤਿਬਿੰਬ ਹੈ ।
ਪ੍ਰਸ਼ਨ 27.
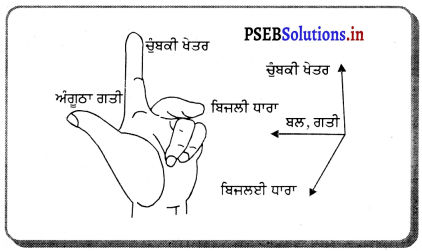
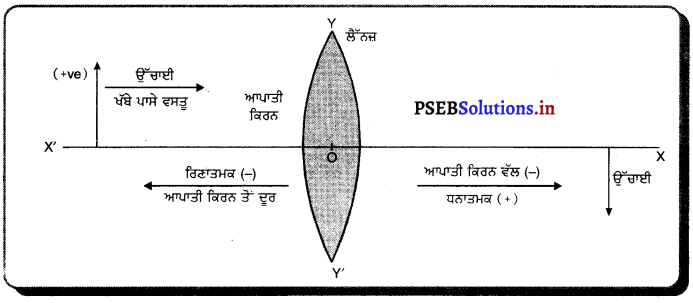
ਗੋਲਾਕਾਰ ਦਰਪਣਾਂ ਦੇ ਲਈ ਨਵੀਆਂ ਕਾਰਟੀਸ਼ੀਅਨ ਚਿੰਨ੍ਹ ਪਰੰਪਰਾਵਾਂ ਦੀ ਰਚਨਾ ਕਰੋ । .
ਜਾਂ
ਗੋਲਾਕਾਰ ਦਰਪਣਾਂ ਦੁਆਰਾ ਪਰਾਵਰਤਨ ਲਈ ਕਿਹੜੀਆਂ ਚਿੰਨ੍ਹ ਪਰੰਪਰਾਵਾਂ ਵਰਤੀਆਂ ਜਾਂਦੀਆਂ ਹਨ ?
ਉੱਤਰ-
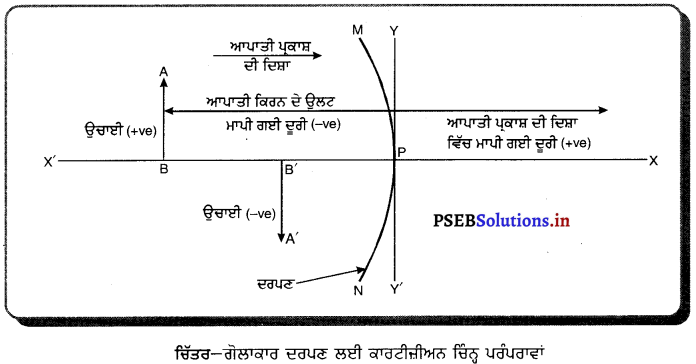
ਨਵੀਆਂ ਕਾਰਟੀਜ਼ੀਅਨੀ ਚਿੰਨ੍ਹ ਪਰੰਪਰਾਵਾਂ (New Cartesian Sign Conversions)-
- ਵਸਤੂ ਜਾਂ ਬਿੰਬ ਦਰਪਣ ਦੇ ਖੱਬੇ ਪਾਸੇ ਹੁੰਦਾ ਹੈ ਅਤੇ ਵਸਤੂ ਤੋਂ ਚਲਣ ਵਾਲੀਆਂ ਆਪਾਤੀ ਕਿਰਨਾਂ ਖੱਬਿਓ ਸੱਜੇ ਪਾਸੇ ਵੱਲ ਨੂੰ ਜਾਂਦੀਆਂ ਹਨ ।
- ਸਾਰੀਆਂ ਦੂਰੀਆਂ ਗੋਲਾਕਾਰ ਦਰਪਣ ਦੇ ਸੀਰਸ਼ (ਜਾਂ ਧਰੁਵ (Pole) ਤੋਂ ਮਾਪੀਆਂ ਜਾਂਦੀਆਂ ਹਨ ।
- ਆਪਾਤੀ ਪ੍ਰਕਾਸ਼ ਦੀ ਦਿਸ਼ਾ ਵਿੱਚ ਮਾਪੀਆਂ ਦੂਰੀਆਂ ਨੂੰ ਧਨਾਤਮਕ ਅਤੇ ਆਪਾਤੀ ਪ੍ਰਕਾਸ਼ ਕਰਨ ਦੀ ਦਿਸ਼ਾ ਦੇ ਉਲਟ ਦਿਸ਼ਾ ਵਿੱਚ ਮਾਪੀਆਂ ਜਾਣ ਵਾਲੀਆਂ ਦੂਰੀਆਂ ਨੂੰ ਰਿਣਾਤਮਕ ਮੰਨਿਆ ਜਾਂਦਾ ਹੈ।
- ਦਰਪਣਾਂ ਦੇ ਮੁੱਖ ਧੁਰੇ ਦੇ ਸਮਕੋਣੀ ਉੱਪਰ ਵੱਲ ਮਾਪੀਆਂ ਜਾਣ ਵਾਲੀਆਂ ਉੱਚਾਈਆਂ ਨੂੰ ਧਨਾਤਮਕ ਅਤੇ ਇਸ ਦੇ ਉਲਟ ਹੇਠਾਂ ਵੱਲ ਉੱਚਾਈਆਂ ਨੂੰ ਰਿਣਾਤਮਕ ਮੰਨਿਆ ਜਾਂਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 28.
ਉੱਤਲ ਦਰਪਣ ਦੁਆਰਾ ਬਣਾਏ ਤਿਬਿੰਬ ਦੀ ਸਥਿਤੀ, ਆਕਾਰ (ਸਾਇਜ਼) ਅਤੇ ਪ੍ਰਕਿਰਤੀ ਲਈ ਸਾਰਨੀ ਬਣਾਓ ।
ਉੱਤਰ-
| ਵਸਤੂ ਦੀ ਸਥਿਤੀ |
ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਸਥਿਤੀ |
ਪ੍ਰਤਿਬਿੰਬ ਦਾ ਆਕਾਰ |
ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਪ੍ਰਕਿਰਤੀ |
| (i) ਅਨੰਤ ਤੇ |
ਦਰਪਣ ਦੇ ਪਿੱਛੇ ਫੋਕਸ F ਤੇ |
ਬਿੰਦੂ ਦੇ ਬਰਾਬਰ |
ਆਭਾਸੀ ਅਤੇ ਸਿੱਧਾ |
| (ii) ਅਨੰਤ ਅਤੇ ਦਰਪਣ ਦੇ ਧਰੁੱਵ P ਦੇ ਵਿਚਕਾਰ |
ਦਰਪਣ ਦੇ ਪਿੱਛੇ P ਅਤੇ F ਦੇ ਵਿਚਕਾਰ |
ਬਹੁਤ ਛੋਟਾ |
ਆਭਾਸੀ ਅਤੇ ਸਿੱਧਾ |
ਪ੍ਰਸ਼ਨ 29.
ਦੋ ਸਮਤਲ ਦਰਪਣਾਂ ਨੂੰ ਕਿਵੇਂ ਵਿਵਸਥਿਤ ਕੀਤਾ ਜਾਵੇ ਕਿ ਪਰਾਵਰਤਿਤ ਕਿਰਨ ਹਮੇਸ਼ਾ ਆਪਾਤੀ ਕਿਰਨ ਦੇ ਸਮਾਨੰਤਰ ਹੋਵੇ ?
ਉੱਤਰ-
ਪਰਾਵਰਤਿਤ ਕਿਰਨ ਹਮੇਸ਼ਾ ਆਪਾਤੀ ਕਿਰਨ ਦੇ ਸਮਾਨੰਤਰ ਹੋਵੇਗੀ ਜੇਕਰ ਦੋ ਸਮਤਲ ਦਰਪਣਾਂ ਨੂੰ ਆਪਸ ਵਿੱਚ ਇੱਕ ਦੂਜੇ ਦੇ ਲੰਬਰੂਪ ਵਿੱਚ ਵਿਵਸਥਿਤ ਕੀਤਾ ਜਾਵੇ ਜਿਵੇਂ ਕਿ ਚਿੱਤਰ ਵਿੱਚ ਦਰਸਾਇਆ ਗਿਆ ਹੈ ।

ਪ੍ਰਸ਼ਨ 30.
ਭਿੰਡਰਾਓ ਪਰਾਵਰਤਨ (Diffused Reflection) ਤੋਂ ਤੁਹਾਡਾ ਕੀ ਭਾਵ ਹੈ ?
ਉੱਤਰ-
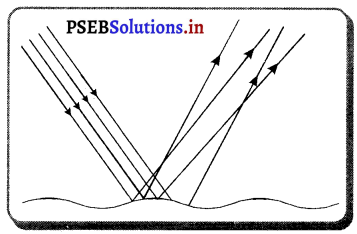
ਖਿੰਡਰਾਓ ਪਰਾਵਰਤਨ-ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਦੀਆਂ ਸਮਾਨੰਤਰ ਕਿਰਨਾਂ ਕਿਸੇ ਅਜਿਹੇ ਤਲ ਤੇ ਟਕਰਾਉਂਦੀਆਂ ਹਨ ਜਿਹੜਾ ਅਸਮਤਲ (ਖੁਰਦਰਾ) ਹੋਵੇ ਤਾਂ ਪ੍ਰਕਾਸ਼ ਦੀਆਂ ਪਰਾਵਰਤਿਤ ਕਿਰਨਾਂ ਦਾ ਇੱਕ ਵੱਡਾ ਭਾਗ ਅਨਿਯਮਿਤ ਰੂਪ ਵਿੱਚ ਖਿੰਡਰ ਜਾਂਦਾ ਹੈ । ਅਜਿਹੇ ਪਰਾਵਰਤਨ ਨੂੰ ਭਿੰਡਰਾਓ ਪਰਾਵਰਤਨ ਕਹਿੰਦੇ ਹਨ ।
ਉਦਾਹਰਨ – ਕਿਤਾਬ ਦੇ ਅੱਖਰਾਂ ਦਾ ਜਾਂ ਬਲੈਕ ਬੋਰਡ ਦੇ ਅੱਖਰਾਂ ਦਾ ਪੜਿਆ ਜਾਣਾ ਭਿੰਡਰਾਓ ਪਰਾਵਰਤਨ ਕਾਰਨ ਹੀ ਸੰਭਵ ਹੈ ।
ਪ੍ਰਸ਼ਨ 31.
ਪ੍ਰਕਾਸ਼ੀ ਮਾਧਿਅਮ ਕਿਸਨੂੰ ਆਖਦੇ ਹਨ ? ਇਹ ਕਿੰਨੇ ਤਰ੍ਹਾਂ ਦੇ ਹੁੰਦੇ ਹਨ ?
ਉੱਤਰ-
ਪ੍ਰਕਾਸ਼ੀ ਮਾਧਿਅਮ – ਜਿਸ ਭੌਤਿਕ ਮਾਧਿਅਮ ਵਿੱਚੋਂ ਪ੍ਰਕਾਸ਼ ਆਸਾਨੀ ਨਾਲ ਲੰਘ ਸਕਦਾ ਹੈ, ਉਸਨੂੰ ਪ੍ਰਕਾਸ਼ੀ ਮਾਧਿਅਮ ਕਹਿੰਦੇ ਹਨ । ਜਿਵੇਂ-ਹਵਾ, ਪਾਣੀ, ਕੱਚ ਆਦਿ ।
ਮਾਧਿਅਮ ਹੇਠ ਲਿਖੇ ਤਿੰਨ ਕਿਸਮ ਦਾ ਹੁੰਦਾ ਹੈ-
- ਪਾਰਦਰਸ਼ੀ ਮਾਧਿਅਮ – ਉਹ ਮਾਧਿਅਮ ਜਿਸ ਵਿੱਚੋਂ ਪ੍ਰਕਾਸ਼ ਦੀਆਂ ਕਿਰਨਾਂ ਆਸਾਨੀ ਨਾਲ ਲੰਘ ਸਕਣ ਅਤੇ ਦੂਜੇ ਪਾਸੇ ਪਈਆਂ ਹੋਈਆਂ ਵਸਤੂਆਂ ਨੂੰ ਸਪੱਸ਼ਟ ਦਿਖਾਈ ਦੇਣ, ਪਾਰਦਰਸ਼ੀ ਮਾਧਿਅਮ ਆਖਦੇ ਹਨ , ਜਿਵੇਂ-ਸਾਫ਼ ਪਾਣੀ, ਕੱਚ ਆਦਿ ।
- ਅਪਾਰਦਰਸ਼ੀ ਮਾਧਿਅਮ – ਉਹ ਮਾਧਿਅਮ ਜਿਸ ਵਿੱਚੋਂ ਪ੍ਰਕਾਸ਼ ਦੀਆਂ ਕਿਰਨਾਂ ਨਾ ਲੰਘ ਸਕਣ ਅਤੇ ਦੂਜੇ ਪਾਸੇ ਪਈਆਂ ਹੋਈਆਂ ਵਸਤੂਆਂ ਦਿਖਾਈ ਨਾ ਦੇਣ, ਅਪਾਰਦਰਸ਼ੀ ਮਾਧਿਅਮ ਕਹਾਉਂਦਾ ਹੈ , ਜਿਵੇਂ-ਇੱਟ, ਗੱਤਾ, ਲੋਹੇ ਦੀ ਚਾਦਰ ਆਦਿ ।
- ਪਾਰਭਾਸੀ (ਅਲਪ-ਪਾਰਦਰਸ਼ੀ) ਮਾਧਿਅਮ – ਜਿਸ ਮਾਧਿਅਮ ਵਿੱਚੋਂ ਪ੍ਰਕਾਸ਼ ਦੀਆਂ ਕਿਰਨਾਂ ਘੱਟ ਮਾਤਰਾ ਵਿੱਚ ਲੰਘ ਸਕਣ ਅਤੇ ਦੂਜੇ ਪਾਸੇ ਪਈਆਂ ਵਸਤੂਆਂ ਧੁੰਦਲੀਆਂ ਦਿਖਾਈ ਦੇਣ, ਪਾਰਭਾਸੀ ਮਾਧਿਅਮ ਕਹਾਉਂਦਾ ਹੈ , ਜਿਵੇਂ ਧੁੰਦਲਾ ਕੱਚ, ਤੇਲ ਲੱਗਿਆ ਕਾਗ਼ਜ਼ ਆਦਿ ।
ਪ੍ਰਸ਼ਨ 32.
ਘਣਤਾ ਦੇ ਪੱਖੋਂ ਮਾਧਿਅਮ ਕਿੰਨੀ ਕਿਸਮ ਦੇ ਹੁੰਦੇ ਹਨ ?
ਉੱਤਰ-
ਘਣਤਾ ਦੇ ਪੱਖੋਂ ਮਾਧਿਅਮ ਦੋ ਕਿਸਮ ਹੁੰਦੇ ਹਨ-
- ਵਿਰਲ ਮਾਧਿਅਮ – ਘੱਟ ਘਣਤਾ ਵਾਲੇ ਮਾਧਿਅਮ ਨੂੰ ਵਿਰਲ ਮਾਧਿਅਮ ਕਹਿੰਦੇ ਹਨ ।
ਉਦਾਹਰਨ – ਹਵਾ ।
- ਸੰਘਣਾ ਮਾਧਿਅਮ – ਵੱਧ ਘਣਤਾ ਵਾਲੇ ਮਾਧਿਅਮ ਨੂੰ ਸੰਘਣਾ ਮਾਧਿਅਮ ਕਹਿੰਦੇ ਹਨ ।
ਉਦਾਹਰਨ – ਕੱਚ ।
ਪ੍ਰਸ਼ਨ 33.
ਵਿਰਲ ਮਾਧਿਅਮ ਤੋਂ ਸੰਘਣੇ ਮਾਧਿਅਮ ਵਿੱਚ ਦਾਖ਼ਲ ਹੁੰਦੇ ਸਮੇਂ ਮਾਧਿਅਮ ਦੀ ਵੱਧ ਸੰਘਣਤਾ ਦਾ ਪ੍ਰਕਾਸ਼ ਕਿਰਨ ਤੇ ਕੀ ਪ੍ਰਭਾਵ ਪੈਂਦਾ ਹੈ ? ਚਿੱਤਰ ਦੁਆਰਾ ਸਮਝਾਓ ।
ਜਾਂ
ਅਪਵਰਤਨ ਵਿੱਚ ਸੰਘਣਤਾ ਦਾ ਅਪਵਰਤਿਤ ਕਰਨ ਦੇ ਝੁਕਾਓ ਤੇ ਕੀ ਪ੍ਰਭਾਵ ਪੈਂਦਾ ਹੈ ? ਚਿੱਤਰ ਦੁਆਰਾ ਸਮਝਾਓ ।
ਉੱਤਰ-
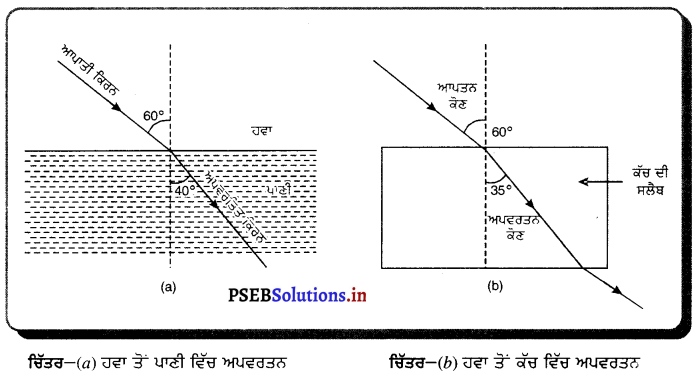
ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਦੀ ਕਿਰਨ ਵਿਰਲ ਤੋਂ ਸੰਘਣ ਮਾਧਿਅਮ ਵਿੱਚ ਪ੍ਰਵੇਸ਼ ਕਰਦੀ ਹੈ ਤਾਂ ਇਹ ਅਭਿਲੰਬ ਵੱਲ ਮੁੜ ਜਾਂਦੀ ਹੈ । ਮਾਧਿਅਮ ਜਿੰਨਾ ਜ਼ਿਆਦਾ ਸੰਘਣਾ ਹੋਵੇਗਾ, ਪ੍ਰਕਾਸ਼ ਕਿਰਨ ਉਨੀ ਹੀ ਅਧਿਕ ਅਭਿਲੰਬ ਵੱਲ ਮੁੜੇਗੀ । ਹੇਠਾਂ ਦਿੱਤੇ ਗਏ ਚਿੱਤਰ ਵਿੱਚ ਪ੍ਰਕਾਸ਼ ਦੀ ਕਿਰਨ ਹਵਾ ਤੋਂ ਪਾਣੀ ਵਿੱਚ ਅਤੇ ਹਵਾ ਤੋਂ ਕੱਚ ਵਿੱਚ ਪ੍ਰਵੇਸ਼ ਕਰਦੀ ਹੋਈ ਦਿਖਾਈ ਦਿੰਦੀ ਹੈ । ਕਿਰਨ ਦਾ ਝੁਕਾਓ ਪਾਣੀ ਦੀ ਤੁਲਨਾ ਵਿੱਚ ਕੱਚ ਵਿੱਚ ਅਧਿਕ ਹੈ ਕਿਉਂਕਿ ਕੱਚ, ਪਾਣੀ ਦੀ ਤੁਲਨਾ ਵਿੱਚ ਅਧਿਕ ਸੰਘਣਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 34.
ਪਾਣੀ ਤੋਂ ਕੱਚ ਵਿੱਚ ਦਾਖ਼ਲ ਹੋਣ ਤੇ ਪ੍ਰਕਾਸ਼ ਦੀ ਚਾਲ ਵਿੱਚ ਕੀ ਪਰਿਵਰਤਨ ਹੁੰਦਾ ਹੈ ?
ਉੱਤਰ-
ਪਾਣੀ, ਕੱਚ ਦੀ ਤੁਲਨਾ ਵਿੱਚ ਵਿਰਲ ਮਾਧਿਅਮ ਹੈ, ਇਸ ਲਈ ਪਕਾਸ਼ ਜਦੋਂ ਪਾਣੀ ਤੋਂ ਕੱਚ ਵਿੱਚ ਪਵੇਸ਼ ਕਰਦਾ ਹੈ ਤਾਂ ਪ੍ਰਕਾਸ਼ ਦੀ ਚਾਲ ਘੱਟ ਹੋ ਜਾਂਦੀ ਹੈ ਅਤੇ ਪ੍ਰਕਾਸ਼ ਅਭਿਲੰਬ ਵੱਲ ਮੁੜ ਜਾਂਦਾ ਹੈ । ਇਸ ਅਵਸਥਾ ਵਿੱਚ ਆਪਨ ਕੋਣ (i) ਅਪਵਰਤਨ ਕੋਣ (r) ਤੋਂ ਵੱਡਾ ਹੁੰਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 35.
ਜੇਕਰ ਪ੍ਰਕਾਸ਼ ਦੀ ਕਿਰਨ ਕੱਚ ਤੋਂ ਪਾਣੀ ਵਿੱਚ ਪ੍ਰਵੇਸ਼ ਕਰੇ ਤਾਂ ਪ੍ਰਕਾਸ਼ ਦੀ ਕਿਰਨ ਅਭਿਲੰਬ ਵੱਲ ਮੁੜੇਗੀ ਜਾਂ ਫਿਰ ਅਭਿਲੰਬ ਤੋਂ ਪਰੇ ਹੋਵੇਗੀ ?
ਉੱਤਰ-
ਇਸ ਅਵਸਥਾ ਵਿੱਚ ਪ੍ਰਕਾਸ਼ ਸੰਘਨ ਮਾਧਿਅਮ (ਕੱਚ) ਤੋਂ ਪਾਣੀ ਵਿਰਲਾ ਮਾਧਿਅਮ ਵਿੱਚ ਪ੍ਰਵੇਸ਼ ਕਰ ਰਹੀ ਹੈ ਜਿਸ ਤੋਂ ਅਪਵਰਤਨ ਹੋਣ ਤੇ ਅਭਿਲੰਬ ਤੋਂ ਦੂਰ ਹਟੇਗੀ । ਇਸ ਅਵਸਥਾ ਵਿੱਚ ਆਪਨ ਕੋਣ (i) ਅਪਵਰਤਨ ਕੋਣ ਤੋਂ ਘੱਟ ਹੋਵੇਗਾ ਅਤੇ ਪਾਣੀ ਵਿੱਚ ਪ੍ਰਕਾਸ਼ ਦੀ ਚਾਲ ਅਧਿਕ ਹੋ ਜਾਵੇਗੀ ।

ਪ੍ਰਸ਼ਨ 36.
ਵਾਸਤਵਿਕ ਅਤੇ ਆਭਾਸੀ ਗਹਿਰਾਈ ਦੇ ਸੰਦਰਭ ਵਿੱਚ ਅਪਵਰਤਨ ਅੰਕ ਗਿਆਤ ਕਰੋ !
ਉੱਤਰ-
ਇਹ ਸਾਧਾਰਨ ਗਿਆਨ ਹੈ ਕਿ ਪਾਣੀ ਦੀ ਤਾਲਾਬ ਵਿੱਚ ਗਹਿਰਾਈ ਘੱਟ ਪ੍ਰਤੀਤ ਹੁੰਦੀ ਹੈ । ਤਾਲਾਬ ਦਾ ਤਲ ਕੁੱਝ ਉੱਪਰ ਉੱਠਿਆ ਹੋਇਆ ਜਾਪਦਾ ਹੈ । ਮੰਨ ਲਓ ਇੱਕ ਵਸਤੁ ਤਾਲਾਬ ਦੀ ਗਹਿਰਾਈ A ਤੇ ਪਈ ਹੋਈ ਹੈ । ਇੱਕ ਕਿਰਨ A ਤੋਂ AB ਲੰਬ ਰੂਪ ਵਿੱਚ ਪਾਣੀ ਦੀ ਸਤਹਿ ਨੂੰ ਟਕਰਾ ਕੇ BD ਵੱਲ ਜਾਂਦੀ ਹੈ । A ਤੋਂ ਇੱਕ ਹੋਰ ਕਿਰਨ c ਤੇ ਆਪਨ ਕੋਣ ∠i ਬਣਾ ਕੇ ਅਭਿਲੰਬ ਵੱਲ ਮੁੜ ਜਾਂਦੀ ਹੈ ਜਿਸ ਤੋਂ A ਆਪਣੀ ਥਾਂ ਤੇ ਨਾ ਰਹਿ ਕੇ A’ ਤੇ ਪ੍ਰਤੀਤ ਹੁੰਦੀ ਹੈ ।
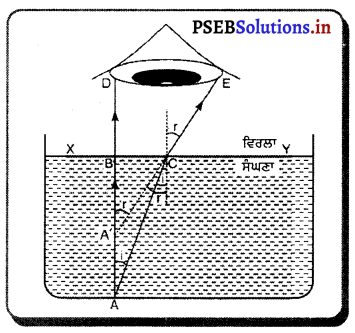
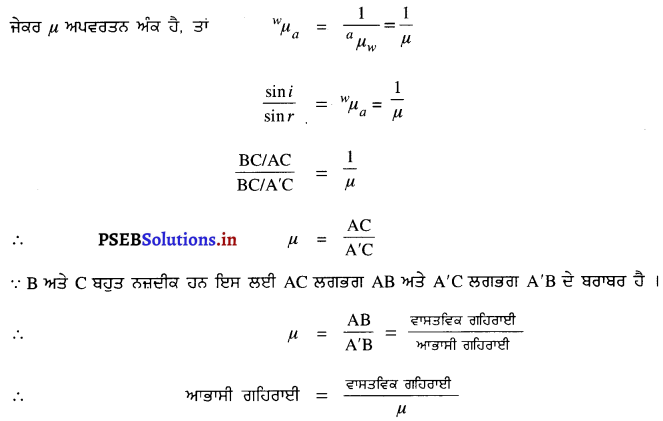
ਪ੍ਰਸ਼ਨ 37.
ਪਾਣੀ ਵਿੱਚ ਡੁੱਬੀ ਹੋਈ ਲੱਕੜੀ (ਪੈਨਸਿਲ) ਮੁੜੀ ਹੋਈ ਪ੍ਰਤੀਤ ਹੁੰਦੀ ਹੈ । ਕਿਉਂ ?
ਉੱਤਰ-
ਮੰਨ ਲਓ ਪਾਣੀ ਵਿੱਚ ਲੱਕੜੀ (ਪੈਂਨਸਿਲ) ਦਾ ਸਿੱਧਾ ਟੁਕੜਾ ਡੁਬੋਇਆ ਗਿਆ ਹੈ ਜੋ ਪ੍ਰਕਾਸ਼ ਅਪਵਰਤਨ ਕਾਰਨ ਮੁੜਿਆ ਹੋਇਆ ਪ੍ਰਤੀਤ ਹੋਵੇਗਾ । ਜਿਵੇਂ ਹੀ ਪ੍ਰਕਾਸ਼ ਦੀ ਕਿਰਨ ਬਿੰਦੁ A ਤੋਂ ਸੰਘਣ ਮਾਧਿਅਮ ਤੋਂ ਵਿਰਲ ਮਾਧਿਅਮ ਵਿੱਚ ਪ੍ਰਵੇਸ਼ ਕਰਦੀ ਹੈ ਤਾਂ ਉਹ ਲੰਬ ਤੋਂ ਪਰੇ ਮੁੜ ਜਾਂਦੀ ਹੈ । ਇਸ ਤਰ੍ਹਾਂ ਬਿੰਦੂ A ਬਿੰਦੂ A’ ਦੇ ਰੂਪ ਵਿੱਚ ਦਿਖਾਈ cਦੀ ਹੈ। ਜਿਸ ਕਾਰਨ ਲੱਕੜੀ (ਪੈਂਨਸਿਲ) ਦਾ ਟੁਕੜਾ ਮੁੜਿਆ ਹੋਇਆ ਜਾਪਦਾ ਹੈ ।
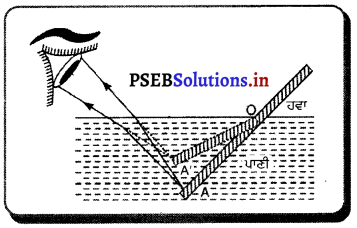
ਪ੍ਰਸ਼ਨ 38.
ਕੀ ਕਾਰਨ ਹੈ ਕਿ ਪਾਣੀ ਨਾਲ ਭਰੇ ਹੋਏ ਟੱਬ ਦੇ ਤਲ ਤੇ ਰੱਖਿਆ ਹੋਇਆ ਸਿੱਕਾ ਸਾਨੂੰ ਥੋੜ੍ਹਾ ਉੱਪਰ ਉੱਠਿਆ ਹੋਇਆ ਪ੍ਰਤੀਤ ਹੁੰਦਾ ਹੈ ? ਚਿੱਤਰ ਦੁਆਰਾ ਦਰਸਾਓ ਕਿ ਪ੍ਰਕਾਸ਼ ਕਿਰਨਾਂ ਕਿਵੇਂ ਚਲਦੀਆਂ ਹਨ ?
ਉੱਤਰ-
ਸਿੱਕੇ ਦਾ ਪਾਣੀ ਵਿੱਚ ਥੋੜ੍ਹਾ ਉੱਪਰ ਉੱਠਿਆ ਹੋਇਆ ਪ੍ਰਤੀਤ ਹੋਣਾ – ਇਸ ਦਾ ਕਾਰਨ ਪ੍ਰਕਾਸ਼ ਅਪਵਰਤਨ ਹੈ । ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਦੀ ਕਿਰਨ ਸੰਘਣੇ ਮਾਧਿਅਮ ਤੋਂ ਚਲ ਕੇ ਵਿਰਲ ਮਾਧਿਅਮ ਵਿੱਚ ਪ੍ਰਵੇਸ਼ ਕਰਦੀ ਹੈ, ਤਾਂ ਅਭਿਲੰਬ ਤੋਂ ਦੂਰ ਹੱਟ ਜਾਂਦੀ ਹੈ ਜਿਸ ਕਾਰਨ ਬਾਹਰੋਂ ਵੇਖਣ ਨਾਲ ਸਾਨੂੰ ਸਿੱਕਾ ਉੱਪਰ ਉੱਠਿਆ ਹੋਇਆ ਦਿਖਾਈ ਦਿੰਦਾ ਹੈ ।
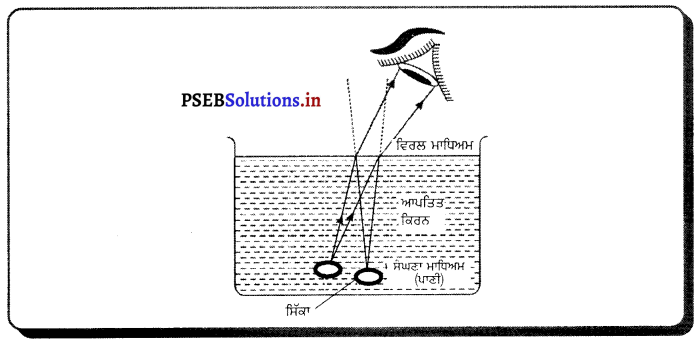
ਪ੍ਰਸ਼ਨ 39.
ਸਨੈੱਲ ਦੇ ਨਿਯਮ ਦੀ ਪਰਿਭਾਸ਼ਾ ਦਿਓ ।
ਉੱਤਰ-
ਸਨੌਲ ਦਾ ਨਿਯਮ (Snell’s Law) – ਅਪਵਰਤਨ ਦੇ ਦੂਜੇ ਨਿਯਮ ਨੂੰ ਸਨੌਲ ਦਾ ਨਿਯਮ ਵੀ ਕਿਹਾ ਜਾਂਦਾ ਹੈ । ਇਸ ਨਿਯਮ ਅਨੁਸਾਰ ਆਪਨ ਕੋਣ ਦੇ sine (sin i) ਅਤੇ ਪਰਾਵਰਤਨ ਕੋਣ ਦੇ sin e (sin r) ਦਾ ਅਨੁਪਾਤ ਨਿਸਚਿਤ ਰਹਿੰਦਾ ਹੈ ।
\(\frac{\sin i}{\sin r}\) = ਸਥਿਰ ਅੰਕ = aμb
ਪ੍ਰਸ਼ਨ 40.
ਅਪਵਰਤਨ ਅੰਕ ਕੀ ਹੁੰਦਾ ਹੈ ? ਇਸਦਾ ਸੰਖਿਆਤਮਕੇ ਫਾਰਮੂਲਾ ਵੀ ਲਿਖੋ ।
ਉੱਤਰ-
ਅਪਵਰਤਨ ਅੰਕ (Refractive Index) – ਨਿਰਵਾਯੂ ਵਿੱਚ ਪ੍ਰਕਾਸ਼ ਦੇ ਵੇਗ ਅਤੇ ਕਿਸੇ ਹੋਰ ਮਾਧਿਅਮ ਵਿੱਚ ਪ੍ਰਕਾਸ਼ ਦੇ ਵੇਗ ਅਨੁਪਾਤ ਨੂੰ ਉਸ ਮਾਧਿਅਮ ਦਾ ਅਪਵਰਤਨ ਅੰਕ ਕਿਹਾ ਜਾਂਦਾ ਹੈ ।

aμb ਨੂੰ ਮਾਧਿਅਮ b ਦਾ ਮਾਧਿਅਮ ਦੇ ਸਾਪੇਖ ਅਪਵਰਤਨ-ਅੰਕ ਕਿਹਾ ਜਾਂਦਾ ਹੈ ਅਰਥਾਤ ਪ੍ਰਕਾਸ਼ ਮਾਧਿਅਮ a ਤੋਂ ਮਾਧਿਅਮ ਠ ਵਿੱਚ ਪ੍ਰਵੇਸ਼ ਕਰਦਾ ਹੈ । ਅਪਵਰਤਨ ਅੰਕ ਦੀ ਕੋਈ ਇਕਾਈ ਨਹੀਂ ਹੁੰਦੀ, ਕਿਉਂਕਿ ਇਹ ਦੋ ਇੱਕ ਕਿਸਮ ਦੀ ਰਾਸ਼ੀਆਂ ਦਾ ਅਨੁਪਾਤ ਹੈ ।

ਪ੍ਰਸ਼ਨ 41.
ਲੈੱਨਜ਼ ਦੀ ਪਰਿਭਾਸ਼ਾ ਦਿਓ । ਲੈੱਨਜ਼ਾਂ ਦੀਆਂ ਕਿਸਮਾਂ ਦੇ ਨਾਮ ਦੱਸੋ ।
ਉੱਤਰ-
ਲੈੱਨਜ਼ (Lens) – ਇਹ ਇੱਕ ਪਾਰਦਰਸ਼ੀ ਅਪਵਰਤਨ ਕਰਨ ਵਾਲੇ ਮਾਧਿਅਮ ਦਾ ਇੱਕ ਹਿੱਸਾ ਹੈ ਜੋ ਦੋ ਗੋਲਾਕਾਰ ਸੜਾਵਾਂ ਨਾਲ ਘਿਰਿਆ ਹੁੰਦਾ ਹੈ ।
ਲੈੱਨਜ਼ ਦੋ ਤਰ੍ਹਾਂ ਦੇ ਹੁੰਦੇ ਹਨ-
(i) ਉੱਤਲ ਲੈੱਨਜ਼
(ii) ਅਵਤਲ ਲੈੱਨਜ਼ ।

ਪ੍ਰਸ਼ਨ 42.
ਲੈੱਨਜ਼ ਲਈ ਇਨ੍ਹਾਂ ਪਦਾਂ ਦੀ ਪਰਿਭਾਸ਼ਾ ਦਿਓ ।
(1) ਪ੍ਰਕਾਸ਼ੀ ਕੇਂਦਰ
(2) ਮੁੱਖ ਧੁਰਾ
(3) ਮੁੱਖ ਫੋਕਸ ਦੀ ।
ਉੱਤਰ-
(1) ਪ੍ਰਕਾਸ਼ੀ ਕੇਂਦਰ (Optical Centre) – ਲੈੱਨਜ਼ ਦੇ ਮੱਧ ਬਿੰਦੂ ਨੂੰ ਪ੍ਰਕਾਸ਼ੀ ਕੇਂਦਰ ਕਿਹਾ ਜਾਂਦਾ ਹੈ । ਇਸ ਬਿੰਦੂ ਵਿੱਚੋਂ ਲੰਘਣ ਵਾਲੀ ਪ੍ਰਕਾਸ਼ ਕਿਰਨ ਬਿਨਾਂ ਮੁੜੇ ਸਿੱਧੀ ਲੰਘ ਜਾਂਦੀ ਹੈ ।
(2) ਮੁੱਖ ਧੁਰਾ (Principal Axis) – ਕਿਸੇ ਲੈੱਨਜ਼ ਦਾ ਮੁੱਖ ਧੁਰਾ ਉਹ ਕਾਲਪਨਿਕ ਰੇਖਾ ਹੈ ਜੋ ਕਿ ਇਸਦੇ ਪ੍ਰਕਾਸ਼ੀ ਕੇਂਦਰ ਵਿੱਚੋਂ ਲੰਘਦੀ ਹੈ ਅਤੇ ਇਹ ਲੈੱਨਜ਼ ਦੇ ਦੋਵੇਂ ਗੋਲਾਕਾਰ ਸੜਾਵਾਂ ਤੇ ਅਭਿਲੰਬ ਹੁੰਦਾ ਹੈ । (ਚਿੱਤਰ) (a) ਵਿੱਚ EF ਉੱਤਲ ਲੈੱਨਜ਼ ਦਾ ਅਤੇ ਚਿੱਤਰ (b) ਵਿੱਚ EF ਅਵਤਲ ਲੈੱਨਜ਼ ਦਾ ਮੁੱਖ ਧੁਰਾ ਹੈ ।
(3) ਮੁੱਖ ਫੋਕਸ (Principal Focus) – ਇਹ ਲੈਂਨਜ਼ ਦੇ ਮੁੱਖ ਧੁਰੇ ਤੇ ਉਹ ਬਿੰਦੂ ਹੈ ਜਿੱਥੇ ਮੁੱਖ ਧੁਰੇ ਦੇ ਸਮਾਨੰਤਰ ਆ ਰਹੀਆਂ ਪ੍ਰਕਾਸ਼ ਕਿਰਨਾਂ ਅਪਵਰਤਨ ਦੇ ਬਾਅਦ ਅਸਲ ਰੂਪ ਵਿੱਚ ਮਿਲਦੀਆਂ ਹਨ (ਉੱਤਲ ਲੈੱਨਜ਼) ਜਾਂ (ਅਵਤਲ ਲੈੱਨਜ਼) ਪਿੱਛੇ ਵੱਲ ਵਧਾਉਣ ਤੇ ਮਿਲਦੀਆਂ ਹੋਈਆਂ ਪ੍ਰਤੀਤ ਹੁੰਦੀਆਂ ਹਨ ।
ਪ੍ਰਸ਼ਨ 43.
ਉੱਤਲ ਲੈੱਨਜ਼ ਦੇ ਅਪਵਰਤਨ ਦੁਆਰਾ ਤਿਬਿੰਬ ਕਰਾਉਣ ਦੇ ਕਿਹੜੇ-ਕਿਹੜੇ ਨਿਯਮ ਹਨ ?
ਉੱਤਰ-
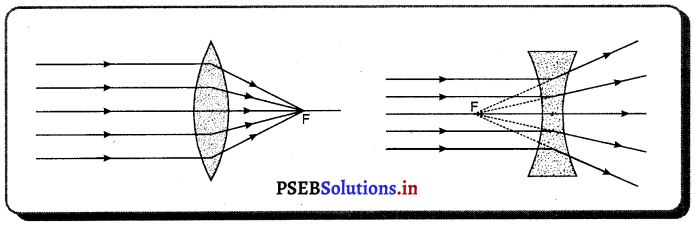
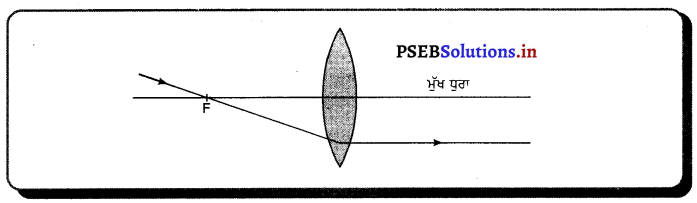
ਉੱਤਲ ਲੈੱਨਜ਼ ਤੋਂ ਅਪਵਰਤਨ ਦੁਆਰਾ ਪ੍ਰਤਿਬਿੰਬ ਬਣਾਉਣ ਦੇ ਨਿਯਮ (Rules for image Formation after Refraction through a Lens) – (1) ਮੁੱਖ ਧੁਰੇ ਦੇ ਸਮਾਨੰਤਰ ਪ੍ਰਕਾਸ਼ ਕਿਰਨ ਅਪਵਰਤਨ ਤੋਂ ਬਾਅਦ ਮੁੱਖ ਫੋਕਸ ਵਿੱਚੋਂ ਲੰਘਦੀ ਹੈ ।
(2) ਮੁੱਖ ਫੋਕਸ ਵਿੱਚੋਂ ਲੰਘ ਰਹੀ ਪ੍ਰਕਾਸ਼ ਕਿਰਨ ਅਪਵਰਤਨ ਤੋਂ ਬਾਅਦ ਮੁੱਖ ਧੁਰੇ ਦੇ ਸਮਾਨੰਤਰ ਹੋ ਜਾਂਦੀ ਹੈ ।
(3) ਲੈੱਨਜ਼ ਦੇ ਪ੍ਰਕਾਸ਼ੀ ਕੇਂਦਰ ਵਿੱਚੋਂ ਲੰਘਦੀ ਪ੍ਰਕਾਸ਼ ਕਰਨ ਵਿਚਲਿਤ ਨਹੀਂ ਹੁੰਦੀ ।
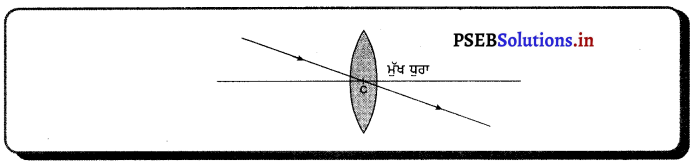
ਪ੍ਰਸ਼ਨ 44.
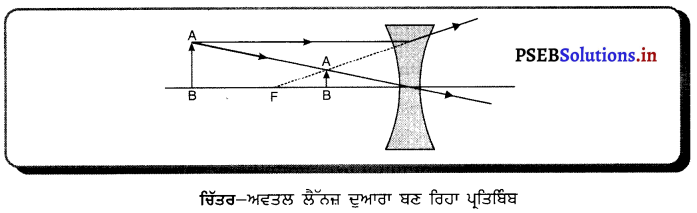
ਕਿਸੇ ਅਵਤਲ ਲੈੱਨਜ਼ ਦੁਆਰਾ ਪ੍ਰਤਿਬਿੰਬ ਕਿਵੇਂ ਬਣਦਾ ਹੈ ? ਕਿਰਨ ਰੇਖਾ-ਚਿੱਤਰ ਬਣਾਓ ( ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਸਥਿਤੀ ਅਤੇ ਪ੍ਰਕਿਰਤੀ ਕਿਹੋ ਜਿਹੀ ਹੋਵੇਗੀ ? | ਉੱਤਰ-
ਅਵਤਲ ਲੈੱਨਜ਼ ਦੇ ਸਾਹਮਣੇ ਰੱਖੀ ਕਿਸੇ ਵਸਤੂ ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਕਿਰਨ ਰੇਖਾ-ਚਿੱਤਰ ਵਿੱਚ ਦਿਖਾਇਆ ਗਿਆ ਹੈ । ਅਵਤਲ ਲੈੱਨਜ਼ ਦੁਆਰਾ ਬਣਾਇਆ ਗਿਆ ਪ੍ਰਤਿਬਿੰਬ ਹਮੇਸ਼ਾ ਲੈੱਨਜ਼ ਦੇ ਪ੍ਰਕਾਸ਼ੀ ਕੇਂਦਰ ਅਤੇ ਫੋਕਸ ਦੇ ਵਿੱਚ ਵਸਤੂ ਵਾਲੇ ਪਾਸੇ ਹੀ ਬਣਦਾ ਹੈ । ਪ੍ਰਤਿਬਿੰਬ ਹਮੇਸ਼ਾ ਸਾਇਜ਼ ਵਿੱਚ ਛੋਟਾ, ਸਿੱਧਾ ਅਤੇ ਆਭਾਸੀ ਬਣਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 45.
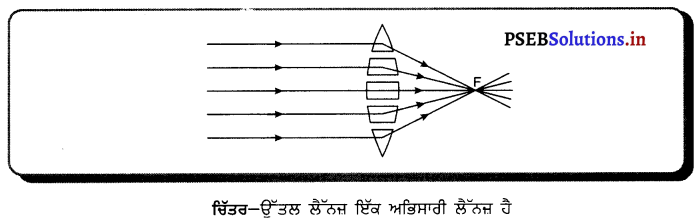
ਚਿੱਤਰ ਦੀ ਸਹਾਇਤਾ ਨਾਲ ਸਮਝਾਓ ਕਿ ਉੱਤਲ ਲੈੱਨਜ਼ ਨੂੰ ਅਭਿਸਾਰੀ ਲੈੱਨਜ਼ ਕਿਉਂ ਕਿਹਾ ਜਾਂਦਾ ਹੈ ?
ਉੱਤਰ-
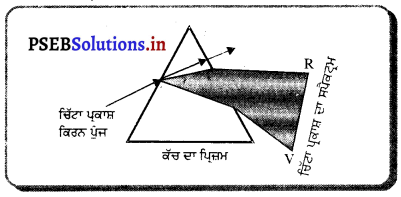
ਉੱਤਲ ਲੈਂਨਜ਼ ਨੂੰ ਪ੍ਰਮਾਂ ਦੇ ਸਮੂਹ ਤੋਂ ਬਣਿਆ ਮੰਨਿਆ ਜਾਂਦਾ ਹੈ, ਜਿਵੇਂ ਕਿ ਚਿੱਤਰ ਵਿੱਚ ਦਿਖਾਇਆ ਹੈ : ਕਿਉਂਕਿ ਪ੍ਰਿਜ਼ਮ ਵਿੱਚੋਂ ਲੰਘਣ ਵਾਲੀ ਕਿਰਨ ਇਸਦੇ ਆਧਾਰ ਵੱਲ ਮੁੜ ਜਾਂਦੀ ਹੈ, ਇਸ ਲਈ ਇਹ ਪ੍ਰਕਾਸ਼ ਨੂੰ ਅਭਿਸਾਰਿਤ (ਇਕੱਠਾ) ਕਰਦਾ ਹੈ । ਇਸ ਲਈ ਇਸਨੂੰ ਅਭਿਸਾਰੀ ਲੈਂਨਜ਼ ਕਿਹਾ ਜਾਂਦਾ ਹੈ ।

ਪ੍ਰਸ਼ਨ 46.
ਚਿੱਤਰ ਦੀ ਸਹਾਇਤਾ ਨਾਲ ਸਮਝਾਓ ਕਿ ਅਵਤਲ ਲੈੱਨਜ਼ ਨੂੰ ਅਪਸਾਰੀ ਲੈਂਨਜ਼ ਕਿਉਂ ਕਿਹਾ ਜਾਂਦਾ ਹੈ ?
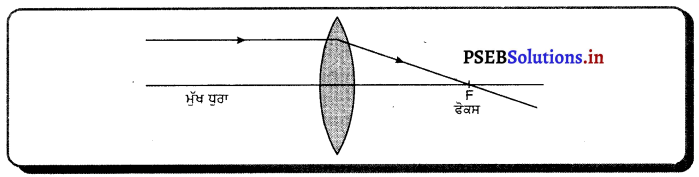
ਉੱਤਰ-
ਅਵਤਲ ਲੈੱਨਜ਼ ਨੂੰ ਦੋ ਪ੍ਰਮਾਂ ਜਿਨ੍ਹਾਂ ਦੇ ਸ਼ੀਰਸ਼ ਕੱਚ ਦੀ ਸਲੈਬ ਦੀ ਆਹਮਣੇ-ਸਾਹਮਣੇ ਵਾਲੀਆਂ ਸਤਾਵਾਂ ਨਾਲ ਸੰਪਰਕ ਕੀਤੇ ਗਏ ਹੋਣ ਦੇ ਸਮਾਨ ਮੰਨਿਆ ਜਾਂਦਾ ਹੈ । ਇਹ ਵਿਵਸਥਾ ਪ੍ਰਕਾਸ਼ ਨੂੰ ਅਪਸਾਰਿਤ ਕਰਨ ਦੇ ਯੋਗ ਹੁੰਦੀ ਹੈ । ਇਸ ਲੈਂਨਜ਼ ਨੂੰ ਅਪਸਾਰੀ ਲੈਂਨਜ਼ ਕਿਹਾ ਜਾਂਦਾ ਹੈ ਕਿਉਂਕਿ ਪ੍ਰਕਾਸ਼ ਕਿਰਨਾਂ ਝੁਕਣ ਦੇ ਬਾਅਦ ਆਧਾਰ ਵੱਲ ਨੂੰ ਜੁੜਦੀਆਂ ਹਨ ਅਤੇ ਅਪਸਰਿਤ ਹੁੰਦੀਆਂ ਦਿਖਾਈ ਦਿੰਦੀਆਂ ਹਨ ।
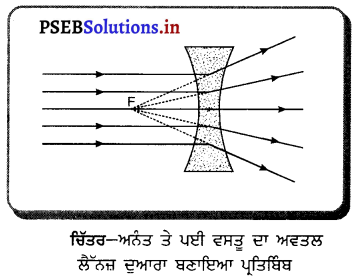
ਪ੍ਰਸ਼ਨ 47.
ਅਵਤਲ ਲੈੱਨਜ਼ ਲਈ, ਜੇ ਵਸਤੂ ਅਨੰਤ ਤੇ ਹੋਵੇ, ਤਾਂ ਪ੍ਰਤਿਬਿੰਬ ਕਿੱਥੇ ਬਣੇਗਾ ਅਤੇ ਇਸ ਦਾ ਸਰੂਪ ਕੀ ਹੋਵੇਗਾ ? ਚਿੱਤਰ ਬਣਾ ਕੇ ਦਰਸਾਓ ।
ਉੱਤਰ-
ਜੇ ਵਸਤੂ ਅਨੰਤ ਤੇ ਹੋਵੇਗੀ, ਤਾਂ ਉਸ ਤੋਂ ਆਉਣ ਵਾਲੀਆਂ ਸਾਰੀਆਂ ਆਪਾਤੀ ਕਿਰਨਾਂ ਇੱਕ-ਦੂਜੇ ਦੇ ਸਮਾਨੰਤਰ ਹੋਣਗੀਆਂ ਅਤੇ ਅਵਤਲ ਲੈੱਨਜ਼ ਵਿੱਚੋਂ ਲੰਘਣ (ਅਪਵਰਤਨ) ਤੋਂ ਬਾਅਦ ਅਪਸਾਰਿਤ ਹੋ ਜਾਣਗੀਆਂ ਜਾਂ ਫੈਲ ਜਾਣਗੀਆਂ। ਇਹ ਸਾਰੀਆਂ ਅਪਵਰਤਿਤ ਕਿਰਨਾਂ ਪਿੱਛੇ ਵੱਲ ਵਧਾਉਣ ਤੇ ਇੱਕ ਬਿੰਦੂ ਤੋਂ ਆਉਂਦੀਆਂ ਹੋਈਆਂ ਪ੍ਰਤੀਤ ਹੁੰਦੀਆਂ ਹਨ । ਇਸ ਬਿੰਦੂ ਤੇ ਪ੍ਰਤਿਬਿੰਬ ਬਣਦਾ ਹੈ । ਇਸ ਬਿੰਦੂ ਨੂੰ ਫੋਕਸ ਕਿਹਾ ਜਾਂਦਾ ਹੈ । ਪ੍ਰਤਿਬਿੰਬ ਆਭਾਸੀ, ਸਿੱਧਾ ਅਤੇ ਸਾਇਜ਼ ਵਿੱਚ ਛੋਟਾ ਹੋਵੇਗਾ । ਚਿੱਤਰ ਵਿੱਚ ਸਾਰੀਆਂ ਚਿੱਤਰ-ਅਨੰਤ ਤੇ ਪਈ ਵਸਤੂ ਦਾ ਅਵਤਲ ਆਪਤਿਤ ਕਿਰਨਾਂ ਅਵਤਲ ਲੈੱਨਜ਼ ਦੇ ਮੁੱਖ ਧੁਰੇ ਦੇ ਸਮਾਨੰਤਰ ਲੈੱਨਜ਼ ਦੁਆਰਾ ਬਣਾਇਆ ਪ੍ਰਤਿਬਿੰਬ ਦਰਸਾਈਆਂ ਗਈਆਂ ਹਨ ।
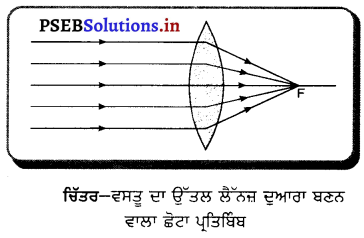
ਪ੍ਰਸ਼ਨ 48.
ਉੱਤਲ ਲੈੱਨਜ਼ ਲਈ, ਵਸਤੂ ਦੀ ਸਥਿਤੀ ਕੀ ਹੋਣੀ ਚਾਹੀਦੀ ਹੈ ਤਾਂ ਜੋ ਪ੍ਰਤਿਬਿੰਬ ਫੋਕਸ ਤੇ ਬਣੇ ਅਤੇ ਬਹੁਤ ਛੋਟਾ ਹੋਵੇ ? ਚਿੱਤਰ ਬਣਾ ਕੇ ਦਰਸਾਓ ।
ਉੱਤਰ-
ਉੱਤਲ ਲੈੱਨਜ਼ ਵਿੱਚ ਪ੍ਰਤਿਬਿੰਬ ਫੋਕਸ ਤੇ ਬਣਨ ਅਤੇ ਆਕਾਰ (ਸਾਇਜ਼) ਵਿੱਚ ਛੋਟਾ ਪ੍ਰਾਪਤ ਕਰਨ ਲਈ ਵਸਤੂ ਨੂੰ ਅਨੰਤ ਤੇ ਅਰਥਾਤ ਲੈੱਨਜ਼ ਤੋਂ ਬਹੁਤ ਦੂਰੀ (ਅਨੰਤ) ਤੇ ਰੱਖਣਾ ਚਾਹੀਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 49.
ਲੈੱਨਜ਼ ਫਾਰਮੂਲਾ ਕੀ ਹੈ ਅਤੇ ਇਸ ਨੂੰ ਵਿਉਂਤਪਤ ਕਰਨ ਲਈ ਕਿਹੜੀਆਂ ਚਿੰਨ੍ਹ ਪਰੰਪਰਾਵਾਂ ਦੀ ਵਰਤੋਂ ਕੀਤੀ ਹੁੰਦੀ ਹੈ ?
ਉੱਤਰ-
ਲੈੱਨਜ਼ ਫਾਰਮੂਲਾ (Lens Formula) – ਲੈੱਨਜ਼ ਫਾਰਮੂਲਾ ਵਸਤੂ ਦੀ ਦੂਰੀ (u), ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਦੂਰੀ (v) ਅਤੇ ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ (f) ਦੇ ਵਿੱਚ ਸੰਬੰਧ ਹੈ ।
\(\frac{1}{v}-\frac{1}{u}=\frac{1}{f}\)
ਚਿੰਨ੍ਹ ਪਰੰਪਰਾਵਾਂ (Sign Conventions)-
- ਸਾਰੀਆਂ ਦੂਰੀਆਂ ਲੈੱਨਜ਼ ਦੇ ਪ੍ਰਕਾਸ਼ੀ ਕੇਂਦਰ ਤੋਂ ਮਾਪੀਆਂ ਜਾਂਦੀਆਂ ਹਨ ।
- ਆਪਾਤੀ ਕਿਰਨ ਦੀ ਦਿਸ਼ਾ ਵਿੱਚ ਮਾਪੀਆਂ ਜਾਣ ਵਾਲੀਆਂ ਦੂਰੀਆਂ ਧਨ (+) ਹੁੰਦੀਆਂ ਹਨ ਜਦੋਂ ਕਿ ਆਪਾਤੀ ਕਿਰਨ ਦੀ ਉਲਟ ਦਿਸ਼ਾ ਵਿੱਚ ਮਾਪੀਆਂ ਜਾਣ ਵਾਲੀਆਂ ਦੂਰੀਆਂ ਰਿਣ ਹੁੰਦੀਆਂ ਹਨ ।
- ਮੁੱਖ ਧੁਰੇ ਤੇ ਅਭਿਲੰਬ ਦੀ ਦਿਸ਼ਾ ਵਿੱਚ ਉੱਪਰ ਵੱਲ ਮਾਪੀਆਂ ਜਾਣ ਵਾਲੀਆਂ ਦੂਰੀਆਂ ਧਨ ਅਤੇ ਹੇਠਾਂ ਵੱਲ ਮਾਪੀਆਂ ਜਾਣ ਵਾਲੀਆਂ ਦੂਰੀਆਂ ਰਿਣ ਹੁੰਦੀਆਂ ਹਨ ।
ਪ੍ਰਸ਼ਨ 50.
ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਕਿਸ ਸਥਿਤੀ ਵਿੱਚ ਇੱਕ ਉੱਤਲ ਲੈੱਨਜ਼ ਆਭਾਸੀ ਅਤੇ ਸਿੱਧਾ ਪ੍ਰਤਿਬਿੰਬ ਬਣਾਉਂਦਾ ਹੈ ? ਇੱਕ ਚਿੱਤਰ ਦੀ ਸਹਾਇਤਾ ਨਾਲ ਆਪਣਾ ਉੱਤਰ ਸਪੱਸ਼ਟ ਕਰੋ ।
ਉੱਤਰ-
ਉੱਤਲ ਲੈੱਨਜ਼ ਦੇ ਪ੍ਰਕਾਸ਼ੀ ਕੇਂਦਰ ਅਤੇ ਫੋਕਸ ਦੇ ਵਿੱਚ ਸਥਿਤ ਵਸਤੂ ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਆਭਾਸੀ, ਸਿੱਧਾ ਅਤੇ ਵਸਤੂ ਤੋਂ ਵੱਡਾ ਬਣਦਾ ਹੈ, ਜਿਵੇਂ ਕਿ ਚਿੱਤਰ ਵਿੱਚ ਦਿਖਾਇਆ ਗਿਆ ਹੈ । ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਸਥਿਤੀ ਲੈੱਨਜ਼ ਦੇ ਉਸੇ ਪਾਸੇ ਹੁੰਦੀ ਹੈ ਜਿਸ ਪਾਸੇ ਵਸਤੂ ਹੈ ।
ਪ੍ਰਸ਼ਨ 51.
ਗੋਲਾਕਾਰ ਲੈਂਨਜ਼ ਲਈ ਵੱਡਦਰਸ਼ਨ ਦੀ ਪਰਿਭਾਸ਼ਾ ਦਿਓ । ਵੱਡਦਰਸ਼ਨ ਦਾ ਮਾਤ੍ਰਿਕ ਕੀ ਹੈ ?
ਜਾਂ
ਰੇਖੀ ਵੱਡਦਰਸ਼ਨ ਦੀ ਪਰਿਭਾਸ਼ਾ ਦਿਓ । ਵੱਡਦਰਸ਼ਨ ਦਾ ਮਾਤ੍ਰਿਕ ਕੀ ਹੈ ?
ਉੱਤਰ-
ਵੱਡਦਰਸ਼ਨ (Magnification) – ਗੋਲਾਕਾਰ ਲੈੱਨਜ਼ ਦਾ ਵੱਡਦਰਸ਼ਨ ਲੈੱਨਜ਼ ਦੁਆਰਾ ਬਣਾਏ ਗਏ ਪ੍ਰਤਿਬਿੰਬ ਦੇ ਆਕਾਰ ਅਤੇ ਵਸਤੂ ਦੇ ਆਕਾਰ ਦਾ ਅਨੁਪਾਤ ਹੁੰਦਾ ਹੈ । ਇਸਨੂੰ ਅ ਨਾਲ ਪ੍ਰਗਟਾਇਆ ਜਾਂਦਾ ਹੈ ।

m ਦਾ ਕੋਈ ਮਾਤ੍ਰਿਕ ਨਹੀਂ ਹੁੰਦਾ ਹੈ ਕਿਉਂਕਿ ਇਹ ਦੋ ਇੱਕੋ ਜਿਹੀਆਂ ਰਾਸ਼ੀਆਂ ਦਾ ਅਨੁਪਾਤ ਹੈ ।

ਪ੍ਰਸ਼ਨ 52.
ਉੱਤਲ ਦਰਪਣ ਅਤੇ ਅਵਤਲ ਦਰਪਣ ਵਿੱਚ ਅੰਤਰ ਸਪੱਸ਼ਟ ਕਰੋ ।
ਉੱਤਰ-
ਉੱਤਲ ਦਰਪਣ ਅਤੇ ਅਵਤਲ ਦਰਪਣ ਵਿੱਚ ਅੰਤਰ-
| ਉੱਤਲ ਦਰਪਣ |
ਅਵਤਲ ਦਰਪਣ |
| (1) ਇਸ ਵਿੱਚ ਪਰਾਵਰਤਨ ਕਰਨ ਵਾਲਾ ਚਮਕੀਲਾ ਤਲ ਬਾਹਰ ਵੱਲ ਉੱਠਿਆ ਹੁੰਦਾ ਹੈ । |
(1) ਇਸ ਵਿੱਚ ਪਰਾਵਰਤਨ ਕਰਨ ਵਾਲਾ ਚਮਕੀਲਾ ਤਲ ਅੰਦਰ ਵੱਲ ਧੱਸਿਆ ਹੁੰਦਾ ਹੈ । |
| (2) ਇਸ ਵਿੱਚ ਪ੍ਰਤਿਬਿੰਬ ਆਭਾਸੀ ਬਣਦਾ ਹੈ । |
(2) ਇਸ ਵਿੱਚ ਪ੍ਰਤਿਬਿੰਬ ਵਾਸਤਵਿਕ ਅਤੇ ਆਭਾਸੀ ਦੋਨੋਂ ਕਿਸਮ ਦੇ ਬਣਦੇ ਹਨ । |
| (3) ਇਸ ਵਿੱਚ ਪ੍ਰਤਿਬਿੰਬ ਸਿੱਧਾ ਬਣਦਾ ਹੈ । |
(3) ਇਸ ਵਿੱਚ ਪ੍ਰਤਿਬਿੰਬ ਸਿੱਧਾ ਅਤੇ ਉਲਟਾ ਦੋਨੋਂ ਪ੍ਰਕਾਰ ਦੇ ਹੁੰਦੇ ਹਨ । |
| (4) ਇਸ ਵਿੱਚ ਪ੍ਰਤਿਬਿੰਬ ਛੋਟਾ ਬਣਦਾ ਹੈ । |
(4) ਇਸ ਵਿੱਚ ਪ੍ਰਤਿਬਿੰਬ ਵੱਡਾ, ਛੋਟਾ ਅਤੇ ਵਸਤੂ ਦੇ ਆਕਾਰ ਦੇ ਬਰਾਬਰ ਤਿੰਨੋਂ ਪ੍ਰਕਾਰ ਦੇ ਬਣਦੇ ਹਨ । |
ਪਸ਼ਨ 53.
ਉੱਤਲ ਲੈਂਨਜ਼ ਅਤੇ ਅਵਤਲ ਲੈੱਨਜ਼ ਵਿੱਚ ਅੰਤਰ ਸਪੱਸ਼ਟ ਕਰੋ ।
ਉੱਤਰ-
ਉੱਤਲ ਲੈੱਨਜ਼ ਅਤੇ ਅਵਤਲ ਲੈੱਨਜ਼ ਵਿੱਚ ਅੰਤਰ-
| ਉੱਤਲ ਲੈੱਨਜ਼ |
ਅਵਤਲ ਲੈੱਨਜ਼ |
| (1) ਇਹ ਵਿਚਕਾਰ ਤੋਂ ਮੋਟਾ ਅਤੇ ਕਿਨਾਰਿਆਂ ਤੋਂ ਪਤਲਾ ਹੁੰਦਾ ਹੈ । |
(1) ਇਹ ਮੱਧ ਤੋਂ ਪਤਲਾ ਅਤੇ ਕਿਨਾਰਿਆਂ ਤੋਂ ਮੋਟਾ ਹੁੰਦਾ ਹੈ । |
| (2) ਇਸ ਵਿੱਚੋਂ ਅੱਖਰ ਵੱਡੇ ਅਤੇ ਮੋਟੇ ਦਿਖਾਈ ਦਿੰਦੇ ਹਨ । |
(2) ਇਸ ਵਿੱਚੋਂ ਅੱਖਰ ਛੋਟੇ ਆਕਾਰ ਦੇ ਦਿਖਾਈ ਦਿੰਦੇ ਹਨ । |
| (3) ਇਹ ਆਪਾਤੀ ਪ੍ਰਕਾਸ਼ ਕਿਰਨਾਂ ਨੂੰ ਇੱਕ ਬਿੰਦੂ ਤੇ ਕੇਂਦ੍ਰਿਤ ਕਰਦਾ ਹੈ । |
(3) ਇਹ ਪ੍ਰਕਾਸ਼ ਕਿਰਨ ਪੁੰਜ ਨੂੰ ਖਿੰਡਰਾਉਂਦਾ ਹੈ । |
| (4) ਵਸਤੂ ਦਾ ਵਾਸਤਵਿਕ ਅਤੇ ਆਭਾਸੀ ਦੋਨੋਂ ਪ੍ਰਕਾਰ ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਬਣਾਉਂਦਾ ਹੈ । |
(4) ਇਹ ਵਸਤੂ ਦਾ ਆਭਾਸੀ ਪ੍ਰਤਿਬਿੰਬ ਬਣਾਉਂਦਾ ਹੈ । |
| (5) ਇਸਦੀ ਫੋਕਸ ਦੂਰੀ ਧਨਾਤਮਕ ਮੰਨੀ ਜਾਂਦੀ ਹੈ । |
(5) ਇਸ ਦੀ ਫੋਕਸ ਦੂਰੀ ਰਿਣਾਤਮਕ ਮੰਨੀ ਜਾਂਦੀ ਹੈ । |
ਪ੍ਰਸ਼ਨ 54.
ਪ੍ਰਕਾਸ਼ ਪਰਾਵਰਤਨ ਅਤੇ ਪ੍ਰਕਾਸ਼ ਅਪਵਰਤਨ ਵਿੱਚ ਕੀ ਅੰਤਰ ਹੈ ?
ਉੱਤਰ-
ਪਰਾਵਰਤਨ ਅਤੇ ਅਪਵਰਤਨ ਵਿੱਚ ਅੰਤਰ-
| ਪਰਾਵਰਤਨ |
ਅਪਵਰਤਨ |
| (1) ਕਿਸੇ ਚਮਕੀਲੀ ਸਤਹਿ ਨੂੰ ਟਕਰਾ ਕੇ ਪ੍ਰਕਾਸ਼ ਕਿਰਨ ਦਾ ਵਾਪਸ ਮੁੜ ਆਉਣਾ ਪ੍ਰਕਾਸ਼ ਪਰਾਵਰਤਨ ਕਹਾਉਂਦਾ ਹੈ । |
(1) ਪ੍ਰਕਾਸ਼ ਦਾ ਇੱਕ ਪਾਰਦਰਸ਼ੀ ਮਾਧਿਅਮ ਤੋਂ ਦੂਜੇ ਪਾਰਦਰਸ਼ੀ ਮਾਧਿਅਮ ਵਿੱਚ ਪ੍ਰਵੇਸ਼ ਹੋਣ ਤੇ ਆਪਣੇ ਪੱਥ ਤੋਂ ਵਿਚਲਿਤ ਹੋਣਾ ਪ੍ਰਕਾਸ਼ ਅਪਵਰਤਨ ਕਹਾਉਂਦਾ ਹੈ । |
| (2) ਇਸ ਵਿੱਚ ਆਪਤਨ ਕੋਣ ਤੇ ਪਰਾਵਰਤਨ ਕੋਣ ਹਮੇਸ਼ਾ ਬਰਾਬਰ ਹੁੰਦੇ ਹਨ । |
(2) ਇਸ ਵਿੱਚ ਆਪਨ ਕੋਣ ਅਤੇ ਅਪਵਰਤਨ ਕੋਣ ਬਰਾਬਰ ਨਹੀਂ ਹੁੰਦੇ । |
| (3) ਪਰਾਵਰਤਨ ਤੋਂ ਬਾਅਦ ਪ੍ਰਕਾਸ਼ ਕਿਰਨਾਂ ਉਸੇ ਮਾਧਿਅਮ ਵਿੱਚ ਵਾਪਸ ਮੁੜ ਆਉਂਦੀਆਂ ਹਨ । |
(3) ਅਪਵਰਤਨ ਤੋਂ ਬਾਅਦ ਪ੍ਰਕਾਸ਼ ਕਿਰਨ ਦੁਜੇ ਮਾਧਿਅਮ ਵਿੱਚ ਚਲੀ ਜਾਂਦੀ ਹੈ । |
ਪ੍ਰਸ਼ਨ 55.
ਬਿਨਾਂ ਛੂਹੇ ਤੁਸੀਂ ਉੱਤਲ ਲੈੱਨਜ਼, ਅਵਤਲ ਲੈੱਨਜ਼ ਅਤੇ ਚੱਕਰਾਕਾਰ ਕੱਚ ਦੀ ਸ਼ੀਟ ਦੀ ਕਿਵੇਂ ਪਛਾਣ ਕਰੋਗੇ ?
ਉੱਤਰ-
ਉੱਤਲ ਲੈੱਨਜ਼, ਅਵਤਲ ਲੈੱਨਜ਼ ਅਤੇ ਕੱਚ ਦੀ ਸ਼ੀਟ ਨੂੰ ਛਪੇ ਹੋਏ ਅੱਖਰਾਂ ਦੇ ਉੱਪਰ ਰੱਖ ਕੇ ਅੱਖ ਵੱਲ ਲਿਆਓ । ਜੇਕਰ ਅੱਖਰਾਂ ਦਾ ਆਕਾਰ (ਸਾਇਜ਼ ਵੱਡਾ ਦਿਖਾਈ ਦੇਵੇ ਤਾਂ ਉਹ ਤਲ ਲੈੱਨਜ਼ ਹੋਵੇਗਾ ਅਤੇ ਜੇਕਰ ਅੱਖਰਾਂ ਦਾ ਆਕਾਰ ਛੋਟਾ ਦਿਖਾਈ ਦੇਵੇ ਤਾਂ ਇਹ ਅਵਤਲ ਲੈੱਨਜ਼ ਹੋਵੇਗਾ ਅਤੇ ਜੇਕਰ ਅੱਖਰਾਂ ਦਾ ਆਕਾਰ ਸਮਾਨ ਰਹੇ ਤਾਂ ਇਹ ਕੱਚ ਦੀ ਸ਼ੀਟ ਹੋਵੇਗੀ ।
ਪ੍ਰਸ਼ਨ 56.
ਇੱਕ-ਦੂਜੇ ਦੇ ਪਰਸਪਰ ਸੰਪਰਕ ਵਿੱਚ ਰੱਖੇ ਹੋਏ ਦੋ ਜਾਂ ਦੋ ਤੋਂ ਵੱਧ ਲੈਂਨਜ਼ਾਂ ਦੀ ਸੰਯੋਜਨ ਸਮਰੱਥਾ ਤੋਂ ਤੁਸੀਂ ਕੀ ਸਮਝਦੇ ਹੋ ?
ਉੱਤਰ-
ਜੇਕਰ ਕਈ ਪਤਲੇ ਲੈੱਨਜ਼ਾਂ ਨੂੰ ਇੱਕ-ਦੂਸਰੇ ਨਾਲ ਜੋੜ ਕੇ ਰੱਖੀਏ ਤਾਂ ਇਸ ਲੈਂਨਜ਼ ਸੰਯੋਜਨ ਦੀ ਕੁੱਲ ਸਮਰੱਥਾ ਉਨ੍ਹਾਂ ਲੈੱਨਜ਼ਾਂ ਦੀ ਵੱਖ-ਵੱਖ ਸਮਰੱਥਾ ਦੇ ਜੋੜ ਦੇ ਬਰਾਬਰ ਹੁੰਦੀ ਹੈ । ਐਨਕ ਬਣਾਉਣ ਵਾਲੇ ਸੰਸ਼ੋਧਿਤ ਲੈੱਨਜ਼ਾਂ ਨੂੰ ਕਈ ਲੈੱਨਜ਼ਾਂ ਦੀ ਸਹਾਇਤਾ ਨਾਲ ਲੋੜੀਂਦੀ ਲੈਂਨਜ਼ ਦੀ ਸਮਰੱਥਾ ਦੀ ਗਣਨਾ ਕਰਦੇ ਹਨ ।
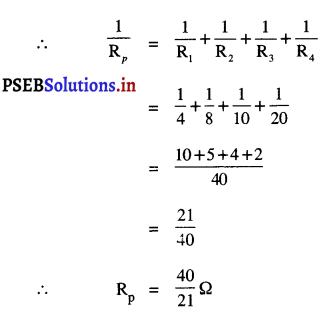
P = P1 + P2 + P3 +……
ਪ੍ਰਸ਼ਨ 57.
ਹੇਠਾਂ ਦਿੱਤੇ ਚਿੱਤਰ ਵਿੱਚ ਕਿਹੜਾ ਦਰਪਣ ਦਰਸਾਇਆ ਹੈ ? ਦਰਪਣ ਦੀ ਤੁਲਨਾ ਵਿੱਚ ਵਸਤੂ ਕਿਸ ਥਾਂ ਤੇ ਪਈ ਹੈ ? ਪ੍ਰਤੀਬਿੰਬ ਦਾ ਇੱਕ ਲੱਛਣ ਦੱਸੋ ।
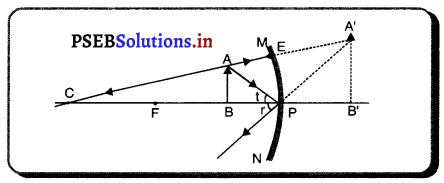
ਉੱਤਰ-
ਚਿੱਤਰ ਵਿੱਚ ਅਵਤਲ ਦਰਪਣ ਦਰਸਾਇਆ ਗਿਆ ਹੈ । ਚਿੱਤਰ ਵਿੱਚ ਵਸਤੂ AB ਦਰਪਣ ਦੇ ਸਾਹਮਣੇ ਫੋਕਸ (F) ਅਤੇ ਧਰੁੱਵ (P) ਦੇ ਵਿਚਕਾਰ ਪਈ ਹੈ ।
ਚਿੱਤਰ ਵਿੱਚ ਬਣ ਰਿਹਾ ਪ੍ਰਤੀਬਿੰਬ A’B’ ਪ੍ਰਕਿਰਤੀ ਵਿੱਚ ਵਸਤੂ ਤੋਂ ਵੱਡਾ, ਸਿੱਧਾ ਅਤੇ ਆਭਾਸੀ ਹੈ । ਪ੍ਰਸ਼ਨ 58. ਗੋਲਾਕਾਰ ਦਰਪਣਾਂ ਦੇ ਦੋ ਉਪਯੋਗ ਲਿਖੋ ।
ਉੱਤਰ-
ਗੋਲਾਕਾਰ ਦਰਪਣ ਦੋ ਕਿਸਮ ਦੇ ਹੁੰਦੇ ਹਨ-
- ਅਵਤਲ ਦਰਪਣ
- ਉੱਤਲ ਦਰਪਣ ।
ਗੋਲਾਕਾਰ ਦਰਪਣਾਂ ਦੇ ਉਪਯੋਗ-
- ਅਵਤਲ ਦਰਪਣ ਪਰਾਵਰਤਕ ਦੇ ਰੂਪ ਵਜੋਂ ਪਰਾਵਰਤਕ ਦੂਰਦਰਸ਼ੀ ਵਿੱਚ ਵਰਤਿਆ ਜਾਂਦਾ ਹੈ ।
- ਉੱਤਲ ਦਰਪਣ ਡਰਾਈਵਰਾਂ ਦੁਆਰਾ ਪਿੱਛੇ ਆ ਰਹੀ ਟ੍ਰੈਫਿਕ ਦਾ ਵਧੇਰੇ ਦ੍ਰਿਸ਼ਟੀ ਖੇਤਰ ਵੇਖਣ ਲਈ ਪ੍ਰਯੋਗ ਕੀਤਾ ਜਾਂਦਾ ਹੈ ਕਿਉਂਕਿ ਇਸ ਵਿੱਚ ਬਣ ਰਿਹਾ ਪ੍ਰਤੀਬਿੰਬ ਛੋਟਾ, ਸਿੱਧਾ ਅਤੇ ਆਭਾਸੀ ਹੁੰਦਾ ਹੈ ।

ਪ੍ਰਸ਼ਨ 59.
ਹੇਠਾਂ ਦਿੱਤੇ ਚਿੱਤਰ ਵਿੱਚ ਕਿਹੜਾ ਦਰਪਣ ਦਰਸਾਇਆ ਹੈ ? ਦਰਪਣ ਦੀ ਤੁਲਨਾ ਵਿੱਚ ਵਸਤੂ ਨੂੰ ਕਿੱਥੇ ਰੱਖਿਆ ਹੈ ? ਪ੍ਰਤੀਬਿੰਬ ਦੇ ਲੱਛਣ ਲਿਖੋ ।
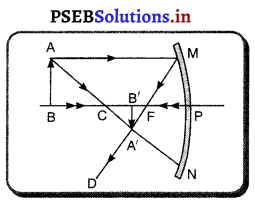
ਉੱਤਰ-
ਚਿੱਤਰ ਵਿੱਚ ਅਵਤਲ ਦਰਪਣ ਦਰਸਾਇਆ ਗਿਆ ਹੈ । ਵਸਤੂ ਦੀ ਦੂਰੀ ਦਰਪਣ ਦੀ ਤੁਲਨਾ ਵਿੱਚ ਵਕੁਤਾ । ਕੇਂਦਰ (C) ਤੋਂ ਪਰੇ ਰੱਖਿਆ ਗਿਆ ਹੈ । ਇਸ ਲਈ ਵਸਤੂ AB ਤੋਂ ਬਣਿਆ ਪ੍ਰਤੀਬਿੰਬ ਵਾਸਤਵਿਕ, ਉਲਟਾ ਅਤੇ ਵਸਤੂ ਦੇ ਆਕਾਰ ਤੋਂ ਛੋਟਾ ਹੈ ।
ਸੰਖਿਆਤਮਕ ਪ੍ਰਸ਼ਨ (Numerical Questions)
ਪ੍ਰਸ਼ਨ 1.
20 cm ਫੋਕਸ-ਦੂਰੀ ਦੇ ਇੱਕ ਅਵਤਲ ਦਰਪਣ ਤੋਂ ਇੱਕ ਵਸਤੂ ਕਿੰਨੀ ਦੂਰ ਰੱਖੀ ਜਾਵੇ, ਤਾਂ ਜੋ ਇਸ ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਦਰਪਣ ਦੇ ਸਾਹਮਣੇ 40 cm ਦੀ ਦੂਰੀ ਤੇ ਬਣੇ ?
ਹੱਲ :
f = -20 cm (ਅਵਤਲ ਦਰਪਣ ਲਈ)
v = – 40 cm (ਆਪਾਤੀ ਕਿਰਨ ਦੀ ਦਿਸ਼ਾ ਦੇ ਉਲਟ ਦਿਸ਼ਾ ਵਿੱਚ ਮਾਪੀ ਗਈ ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਦੂਰੀ)
u = ? (ਅਵਤਲ ਦਰਪਣ ਤੋਂ ਵਸਤੂ ਦੀ ਦੂਰੀ )
ਦਰਪਣ ਫਾਰਮੂਲੇ ਤੋਂ
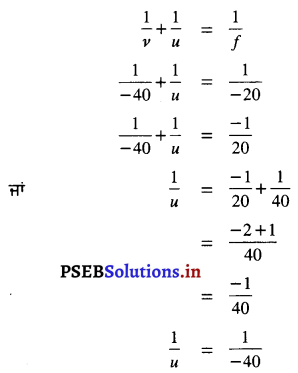
ਜਾਂ u = 40 cm.
ਇਸ ਲਈ ਵਸਤੁ ਅਵਤਲ ਦਰਪਣ ਦੇ ਸਾਹਮਣੇ 40 cm ਦੀ ਦੂਰੀ ਤੇ ਰੱਖੀ ਜਾਵੇ। ਉੱਤਰ
ਪ੍ਰਸ਼ਨ 2.
ਇੱਕ ਅਵਤਲ ਦਰਪਣ ਦਾ ਵਕਤਾ ਅਰਧ-ਵਿਆਸ 15 cm ਹੈ ਅਤੇ ਇੱਕ ਵਸਤੂ ਨੂੰ ਇਸ ਦੇ ਸ਼ੀਰਸ਼ ਤੋਂ 20 cm ਤੇ ਰੱਖਿਆ ਜਾਂਦਾ ਹੈ । ਪ੍ਰਤਿਬਿੰਬ ਦਾ ਸਰੂਪ ਅਤੇ ਸਥਿਤੀ ਪਤਾ ਕਰੋ ।
ਹੱਲ :
ਵਕਤਾ ਅਰਧ-ਵਿਆਸ (R) = -15 cm (ਅਵਤਲ ਦਰਪਣ ਲਈ)
ਅਵਤਲ ਦਰਪਣ ਦੀ ਫੋਕਸ-ਦੂਰੀ (f) = \(\frac{-15}{2}\) cm
ਵਸਤੂ ਦੀ ਅਵਤਲ ਦਰਪਣ ਦੇ ਧਰੁਵ ਤੋਂ ਦੂਰੀ (u) = -20 cm (ਆਪਾਤੀ ਕਿਰਨ ਦੀ ਉਲਟ ਦਿਸ਼ਾ ਵਿੱਚ ਮਾਪਿਆ ਗਿਆ)
ਪ੍ਰਤੀਬਿੰਬ ਦੀ ਅਵਤਲ ਦਰਪਣ ਦੇ ਧਰੁਵ ਤੋਂ ਦੂਰੀ (υ) = ?
ਵੱਡਦਰਸ਼ਨ (m) = ?
ਦਰਪਣ ਫਾਰਮੂਲੇ ਤੋਂ \(\frac{1}{v}+\frac{1}{u}=\frac{1}{f}\)
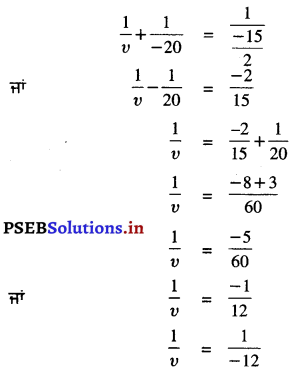
∴ υ = -12
ਅਰਥਾਤ ਤਿਬਿੰਬ ਦਰਪਣ ਦੇ ਸਾਹਮਣੇ 12 cm ਦੀ ਦੂਰੀ ਤੇ ਬਣਦਾ ਹੈ ।
ਹੁਣ ਵੱਡਦਰਸ਼ਨ (m) = \(\frac{-v}{u}\)
= \(\frac{-(-12)}{-20}\)
= \(\frac{-12}{20}\)
= \(\frac{-3}{5}\) = – 0.6
m < 1
ਕਿਉਂਕਿ ਵੱਡਦਰਸ਼ਨ ਦਾ ਮੁੱਲ 1 ਤੋਂ ਘੱਟ ਹੈ ਅਤੇ υ ਪਰਿਣਾਤਮਕ ਹੈ, ਇਸ ਲਈ ਪ੍ਰਤਿਬਿੰਬ ਵਸਤੂ ਦੇ ਸਾਇਜ਼ ਤੋਂ ਛੋਟਾ, ਉਲਟਾ ਅਤੇ ਵਾਸਤਵਿਕ ਹੈ ।
ਪ੍ਰਸ਼ਨ 3.
ਇੱਕ 6 cm ਲੰਬੀ ਵਸਤੂ ਨੂੰ 18 cm ਫੋਕਸ ਦੂਰੀ ਵਾਲੇ ਇੱਕ ਦਰਪਣ ਤੋਂ 10 cm ਦੀ ਦੂਰੀ ਤੇ ਰੱਖਿਆ ਗਿਆ ਹੈ । ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਸਥਿਤੀ ਅਤੇ ਵੱਡਦਰਸ਼ਨ ਪਤਾ ਕਰੋ ।
ਹੱਲ :
u = -10 cm
f = +18 cm (ਉੱਤਲ ਦਰਪਣ ਲਈ)
h1 = + 6 cm (ਮੁੱਖ ਧੁਰੇ ਤੋਂ ਉੱਪਰ ਵੱਲ ਮਾਪਣ ‘ਤੇ)
ਦਰਪਣ ਫਾਰਮੂਲੇ ਤੋਂ, \(\frac{1}{v}+\frac{1}{u}=\frac{1}{f}\)
∴ \(\frac{1}{v}+\frac{1}{-10}=\frac{1}{18}\)
∴ \(\frac{1}{v}=\frac{1}{10}+\frac{1}{18}\)
\(\frac{1}{v}=\frac{18+10}{180}\)
= \(\frac{28}{180}\)
ਜਾਂ υ = + 6.4 cm
ਕਿਉਂਕਿ υ ਧਨਾਤਮਕ ਹੈ, ਇਸ ਲਈ ਪ੍ਰਤਿਬਿੰਬ ਦਰਪਣ ਦੇ ਪਿੱਛੇ 6.4 cm ਦੀ ਦੂਰੀ ‘ਤੇ ਬਣਦਾ ਹੈ ਅਤੇ ਆਭਾਸੀ ਹੈ ।
ਹੁਣ ਵੱਡਦਰਸ਼ਨ, m = \(\frac{-v}{u}\)
= \(\frac{(+6.4)}{-10}\)
= 0.64 < 1
m < 1
ਕਿਉਂਕਿ ਅ ਦਾ ਮੁੱਲ ਇੱਕ ਤੋਂ ਘੱਟ ਹੈ। ਇਸਦਾ ਅਰਥ ਹੈ ਕਿ ਪਤਿਬਿੰਬ ਦਾ ਸਾਈਜ਼ ਛੋਟਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 4.
ਇੱਕ ਅਵਤਲ ਦਰਪਣ ਦਾ ਵਕੁਤਾ ਅਰਧ-ਵਿਆਸ 8 cm ਹੈ ਅਤੇ ਇੱਕ ਵਸਤੂ ਇਸਦੇ ਧਰੁਵ ਤੋਂ 20 cm ਦੂਰੀ ‘ਤੇ ਰੱਖੀ ਜਾਂਦੀ ਹੈ । ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਪ੍ਰਕਿਰਤੀ ਅਤੇ ਸਥਿਤੀ ਪਤਾ ਕਰੋ ।
ਹੱਲ :
u = -20 cm (ਆਪਾਤੀ ਕਿਰਨ ਦੇ ਉਲਟ ਦਿਸ਼ਾ ਵਿੱਚ ਮਾਪੀ ਗਈ ਦੁਰੀ)
R = -8 cm (ਅਵਤਲ ਦਰਪਣ ਲਈ ਫੋਕਸ ਦੂਰੀ ਰਿਣਾਤਮਕ ਹੁੰਦੀ ਹੈ)
ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਪ੍ਰਕਿਰਤੀ = ?
υ = ?
R = 2f,

υ = -5 cm
ਰਿਣਾਤਮਕ ਚਿੰਨ੍ਹ ਇਹ ਦਰਸਾਉਂਦਾ ਹੈ ਕਿ ਪ੍ਰਤਿਬਿੰਬ ਦਰਪਣ ਦੇ ਸਾਹਮਣੇ ਪਾਸੇ 5 cm ਦੀ ਦੂਰੀ ਤੇ ਬਣਦਾ ਹੈ । ਇਸ ਲਈ ਇਹ ਪ੍ਰਤਿਬਿੰਬ ਵਾਸਤਵਿਕ ਅਤੇ ਉਲਟਾ ਹੈ ।

ਪ੍ਰਸ਼ਨ 5.
7.5 cm ਉਚਾਈ ਦੀ ਇੱਕ ਵਸਤੂ 20 cm ਅਰਧ-ਵਿਆਸ ਵਾਲੇ ਉੱਤਲ ਦਰਪਣ ਦੇ ਸਾਹਮਣੇ 40 cm ਦੀ ਦੂਰੀ ਤੇ ਪਈ ਹੈ। ਤਿਬਿੰਬ ਦੀ ਦੂਰੀ, ਸੁਭਾਅ ਅਤੇ ਆਕਾਰ ਗਿਆਤ ਕਰੋ ।
ਹੱਲ :
ਅਸੀਂ ਜਾਣਦੇ ਹਾਂ ਕਿ f = \(\frac{\mathrm{R}}{2}\)
∴ = \(\frac{20}{2}\) = 10 cm
u = -40 cm
(ਆਪਾਤੀ ਕਿਰਨ ਦੀ ਦਿਸ਼ਾ ਦੇ ਉਲਟ ਦਿਸ਼ਾ ਵਿੱਚ ਮਾਪੀ ਗਈ ਦੂਰੀ)
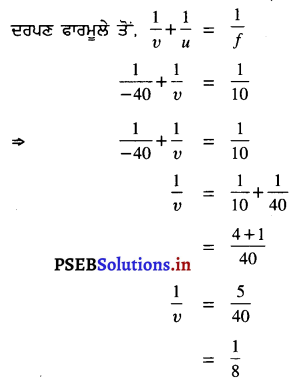
υ = 8 cm
ਕਿਉਂਕਿ υ ਦਾ ਚਿੰਨ੍ਹ ਧਨਾਤਮਕ ਹੈ, ਇਸ ਲਈ ਪ੍ਰਤਿਬਿੰਬ ਸਿੱਧਾ ਅਤੇ ਆਭਾਸੀ ਹੈ ਅਤੇ ਦਰਪਣ ਦੇ ਪਿੱਛੇ 8 cm ਦੀ ਦੂਰੀ ‘ਤੇ ਬਣਦਾ ਹੈ ।
ਹੁਣ ਵੱਡਦਰਸ਼ਨ m = \(\frac{-v}{u}\)
= \(\frac{-8}{-40}\)
= \(\frac{1}{5}\) = 0.2
m < 1
ਕਿਉਂਕਿ m < 1 ਹੈ, ਇਸ ਲਈ ਪ੍ਰਤਿਬਿੰਬ ਦਾ ਸਾਈਜ਼ ਵਸਤੂ ਦੇ ਸਾਈਜ਼ ਦੀ ਤੁਲਨਾ ਵਿੱਚ ਛੋਟਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 6.
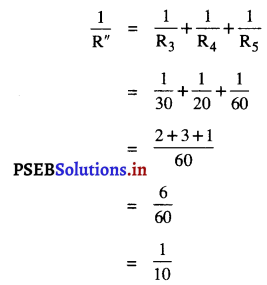
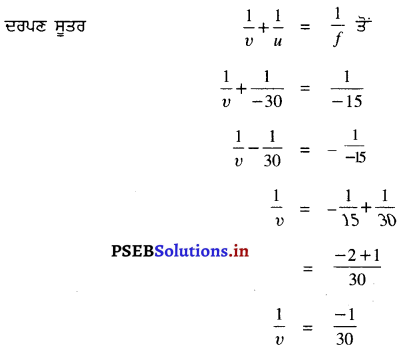
ਇੱਕ ਵਸਤੂ ਦਾ ਸਾਇਜ਼ 6 ਸੈਂ. ਮੀ. ਹੈ । ਇਸਨੂੰ 15 ਸੈਂ. ਮੀ. ਫੋਕਸ ਦੂਰੀ ਵਾਲੇ ਇੱਕ ਉੱਤਲ ਦਰਪਣ ਤੋਂ 9 ਸੈਂ. ਮੀ. ਦੀ ਦੂਰੀ ‘ਤੇ ਰੱਖਿਆ ਜਾਂਦਾ ਹੈ । ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਸਥਿਤੀ ਪਤਾ ਕਰੋ ।
ਹੱਲ :
ਫੋਕਸ ਦੂਰੀ (f) = 15 ਸੈਂ. ਮੀ. (ਉੱਤਲ ਦਰਪਣ ਲਈ)
ਵਸਤੂ ਦੀ ਧਰੁਵ ਤੋਂ ਦੂਰੀ (u) = – 9 ਸੈਂ. ਮੀ.
ਵਸਤੂ ਦਾ ਸਾਈਜ਼ = 6 ਸੈਂ. ਮੀ.

υ = \(\frac{45}{8}\) = 5.62 ਸੈਂ. ਮੀ. ਪ੍ਰਤਿਬਿੰਬ ਵਸਤੂ ਤੋਂ ਦੂਜੀ ਪਾਸੇ ਵੱਲ ਹੋਵੇਗਾ।
ਪ੍ਰਸ਼ਨ 7.
ਉਸ ਉੱਤਲ ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ ਪਤਾ ਕਰੋ ਜਿਸ ਦਾ ਵਰ੍ਹਾ ਅਰਧ-ਵਿਆਸ 32 ਸਮ ਹੈ ?
ਹੱਲ :
ਉੱਤਲ ਲੈੱਨਜ਼ ਦਾ ਵਰ੍ਹਾ ਅਰਧ ਵਿਆਸ (r) = 32 ਸਮ
ਅਸੀਂ ਜਾਣਦੇ ਹਾਂ, r = 2f
ਜਾਂ f = \(\frac{r}{2}\)
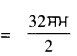
∴ ਉੱਤਲ ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ f = 16 ਸਮ
ਪ੍ਰਸ਼ਨ 8.
ਅਸੀਂ 20 cm ਫੋਕਸ ਦੂਰੀ ਵਾਲੇ ਕਿਸੇ ਪਤਲੇ ਉੱਤਲ ਲੈੱਨਜ਼ ਦੁਆਰਾ ਕਿਸੇ ਵਸਤੂ ਦਾ ਵਾਸਤਵਿਕ, ਉਲਟਾ ਅਤੇ ਸਾਈਜ਼ ਵਿੱਚ ਵਸਤੂ ਦੇ ਬਰਾਬਰ ਪ੍ਰਤਿਬਿੰਬ ਪ੍ਰਾਪਤ ਕਰਨਾ ਚਾਹੁੰਦੇ ਹਾਂ । ਦੱਸੋ ਵਸਤੂ ਨੂੰ ਕਿੱਥੇ ਰੱਖਣਾ ਚਾਹੀਦਾ ਹੈ ? ਇਸ ਪ੍ਰਕਰਣ ਨੂੰ ਦਰਸਾਉਣ ਲਈ ਪ੍ਰਕਾਸ਼ ਕਰਨ ਦਾ ਰੇਖਾ ਚਿੱਤਰ ਖਿੱਚੋ ।
ਹੱਲ :
20 cm ਫੋਕਸ ਦੂਰੀ ਵਾਲੇ ਉੱਤਲ ਲੈੱਨਜ਼ ਦੁਆਰਾ ਵਾਸਤਵਿਕ ਉਲਟਾ ਅਤੇ ਵਸਤੂ ਦੇ ਆਕਾਰ ਦੇ ਬਰਾਬਰ ਪ੍ਰਤਿਬਿੰਬ ਪ੍ਰਾਪਤ ਕਰਨ ਲਈ ਸਾਨੂੰ ਲੈੱਨਜ਼ ਤੋਂ 2F ਦੀ ਦੂਰੀ ਤੇ ਰੱਖਣਾ ਚਾਹੀਦਾ ਹੈ। ਪ੍ਰਤਿਬਿੰਬ ਲੈਂਨਜ਼ ਦੇ ਦੂਜੇ ਪਾਸੇ 2F ਦੂਰੀ ਤੇ ਬਣੇਗਾ ।
∴ ਵਸਤੂ ਦੀ ਲੈਂਨਜ਼ ਤੋਂ ਦੂਰੀ = 2f
= 2 × f
= 2 × 20 cm
= 40 cm
ਪ੍ਰਸ਼ਨ 9.
4.0 cm ਉੱਚਾਈ ਵਾਲੀ ਇੱਕ ਵਸਤੂ 15.0 cm ਫੋਕਸ ਦੂਰੀ ਵਾਲੇ ਅਵਤਲ ਦਰਪਣ ਤੋਂ 30.0 cm ਦੀ ਦੂਰੀ ਤੇ ਰੱਖੀ ਗਈ ਹੈ । ਦਰਪਣ ਤੋਂ ਕਿੰਨੀ ਦੂਰੀ ਤੇ ਇੱਕ ਪਰਦੇ ਨੂੰ ਰੱਖਿਆ ਜਾਏ ਤਾਂ ਜੋ ਵਸਤੂ ਦਾ ਸਪੱਸ਼ਟ ਪ੍ਰਤਿਬਿੰਬ ਪਰਦੇ ਉੱਤੇ ਪ੍ਰਾਪਤ ਹੋ ਸਕੇ ? ਤਿਬਿੰਬ ਦੀ ਪ੍ਰਕਿਰਤੀ ਅਤੇ ਸਾਈਜ਼ ਵੀ ਪਤਾ ਕਰੋ। ਹੱਲ :
ਦਿੱਤਾ ਹੈ, ਵਸਤੂ ਦੀ ਉੱਚਾਈ (ਸਾਈਜ਼), h = 4.0 cm
ਵਸਤੂ ਦੀ ਅਵਤਲ ਦਰਪਣ ਤੋਂ ਦੂਰੀ, u = -30 cm
ਅਵਤਲ ਦਰਪਣ ਦੀ ਫੋਕਸ ਦੂਰੀ, f = -15.0 cm
ਪਰਦੇ ਅਰਥਾਤ ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਦਰਪਣ ਤੋਂ ਦੂਰੀ, υ = ?
ਪ੍ਰਤਿਬਿੰਬ ਦਾ ਸਾਈਜ਼, h = ?
∴ υ = -30 cm
ਅਰਥਾਤ ਪਰਦੇ ਨੂੰ ਦਰਪਣ ਦੇ ਸਾਹਮਣੇ 30 cm ਦੀ ਦੂਰੀ ਤੇ ਰੱਖਣਾ ਚਾਹੀਦਾ ਹੈ ਜਿਸ ਪਾਸੇ ਵਸਤੂ ਪਈ ਹੈ ।
ਪ੍ਰਤਿਬਿੰਬ ਕਿਰਤੀ – ਕਿਉਂਕਿ ਪ੍ਰਤਿਬਿੰਬ ਪਰਦੇ ਤੇ ਪ੍ਰਾਪਤ ਹੋ ਰਿਹਾ ਹੈ ਅਤੇ ਰਿਣਾਤਮਕ ਚਿੰਨ੍ਹ ਦਾ ਹੈ ਇਸ ਲਈ ਪ੍ਰਤਿਬਿੰਬ ਵਾਸਤਵਿਕ ਅਤੇ ਉਲਟਾ ਹੋਵੇਗਾ ।
ਪ੍ਰਤਿਬਿੰਬ ਦਾ ਆਕਾਰ (ਸਾਈਜ਼)-
ਦਰਪਣ ਦੇ ਵੱਡਦਰਸ਼ਨ ਸੂਤਰ ਤੋਂ
m = \(\frac{h^{\prime}}{h}=\frac{-v}{u}\)
ਜਾਂ ਤਿਬਿੰਬ ਦਾ ਆਕਾਰ, h’ = \(\frac{-v}{u}\) × h
= \(\frac{-(-30)}{-30}\) × 4
= \(\frac{30}{-30}[latex] × 4
= – 1 × 4
= -4 cm

ਪ੍ਰਸ਼ਨ 10.
3 cm ਉੱਚੇ ਪ੍ਰਤੀਬਿੰਬ ਨੂੰ 18 cm ਫੋਕਸ ਦੂਰੀ ਵਾਲੇ ਇੱਕ ਅਵਤਲ ਦਰਪਣ ਦੇ ਸਾਹਮਣੇ 9 cm ਦੀ ਦੂਰੀ ਤੇ ਰੱਖਿਆ ਗਿਆ ਹੈ । ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਸਥਿਤੀ, ਪ੍ਰਕਿਰਤੀ ਅਤੇ ਆਕਾਰ ਗਿਆਤ ਕਰੋ ।
ਹੱਲ :
ਦਿੱਤਾ ਹੈ, ਵਸਤੂ ਦੀ ਅਵਤਲ ਦਰਪਣ ਤੋਂ ਦੂਰੀ, u = -9 cm
ਅਵਤਲ ਦਰਪਣ ਦੀ ਫੋਕਸ ਦੂਰੀ, f = -18 cm
ਵਸਤੂ ਦੀ ਉੱਚਾਈ, h = 3 cm
ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਸਥਿਤੀ (ਦਰਪਣ ਤੋਂ ਦੂਰੀ), υ = ?
ਪ੍ਰਤਿਬਿੰਬ ਦਾ ਆਕਾਰ, ਉੱਚਾਈ) h’ = ?
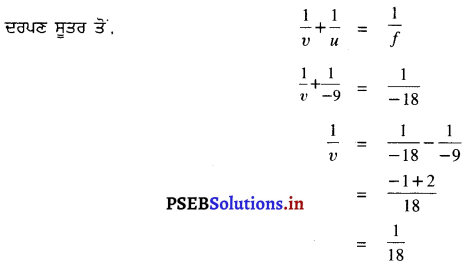
∴ υ = + 18 cm
ਧਨਾਤਮਕ ਚਿੰਨ੍ਹ ਇਹ ਦਰਸਾਉਂਦਾ ਹੈ ਕਿ ਪ੍ਰਤਿਬਿੰਬ ਦਰਪਣ ਦੇ ਪਿੱਛੇ 18 cm ਦੀ ਦੂਰੀ ਤੇ ਬਣੇਗਾ ਇਸ ਲਈ ਇਹ ਆਭਾਸੀ ਅਤੇ ਸਿੱਧਾ ਹੋਵੇਗਾ ।
ਹੁਣ ਦਰਪਣ ਦੇ ਵੱਡਦਰਸ਼ਨ ਤੋਂ, m = [latex]\frac{h^{\prime}}{h}=\frac{v}{u}\)
ਜਾਂ h’ = \(\frac{-v}{u}\) × h
= \(\frac{-(18)}{-9}\) × 3
∴ ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਉੱਚਾਈ (ਆਕਾਰ) = 6 cm
ਪ੍ਰਸ਼ਨ 11.
18 cm ਫੋਕਸ ਦੂਰੀ ਵਾਲੇ ਉੱਤਲ ਲੈੱਨਜ਼ ਤੋਂ ਵਸਤੂ ਨੂੰ ਕਿੰਨੀ ਦੂਰੀ ਤੇ ਰੱਖਿਆ ਜਾਣਾ ਚਾਹੀਦਾ ਹੈ, ਤਾਂ ਜੋ ਇਸ ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਲੈਂਨਜ਼ ਤੋਂ 24 cm ਦੀ ਦੂਰੀ ਤੇ ਬਣੇ ? ਇਸ ਸਥਿਤੀ ਵਿੱਚ ਇਸਦਾ ਵੱਡਦਰਸ਼ਨ ਵੀ ਪਤਾ ਕਰੋ ?
ਹੱਲ :
ਦਿੱਤਾ ਹੈ, ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ, f = + 18 cm
ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਲੈੱਨਜ਼ ਤੋਂ ਦੂਰੀ, υ = + 24 cm
ਵਸਤੂ ਦੀ ਲੈੱਨਜ਼ ਤੋਂ ਦੂਰੀ, u = ?

∴ u = -72 cm
ਰਿਣਾਤਮਕ ਚਿੰਨ੍ਹ ਇਹ ਦਰਸਾਉਂਦਾ ਹੈ ਕਿ ਵਸਤੂ ਨੂੰ ਲੈੱਨਜ਼ ਦੇ ਸਾਹਮਣੇ 72 cm ਦੀ ਦੂਰੀ ‘ਤੇ ਰੱਖਣਾ ਚਾਹੀਦਾ ਹੈ ।
ਵੱਡਦਰਸ਼ਨ m = \(\frac{-v}{u}\)
= \(\frac{-24}{-72}\)
= \(\frac{1}{3}\)
ਪ੍ਰਤਿਬਿੰਬ ਵਾਸਤਵਿਕ, ਉਲਟਾ ਅਤੇ ਆਕਾਰ ਵਿੱਚ ਵਸਤੂ ਦਾ \(\frac{1}{3}\) ਹੋਵੇਗਾ ।
ਪ੍ਰਸ਼ਨ 12.
ਇੱਕ ਉੱਤਲ ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ 25 cm ਹੈ । ਵਸਤੂ ਦੀ ਲੈੱਨਜ਼ ਤੋਂ ਦੂਰੀ ਗਿਆਤ ਕਰੋ, ਜਦਕਿ ਉਸਦਾ ਤਿਬਿੰਬ ਲੈਂਨਜ਼ ਤੋਂ 75 cm ਦੀ ਦੂਰੀ ‘ਤੇ ਬਣਦਾ ਹੈ । ਇਸ ਤਿਬਿੰਬ ਦੀ ਪ੍ਰਕਿਰਤੀ ਕੀ ਹੋਵੇਗੀ ?
ਹੱਲ :
ਦਿੱਤਾ ਹੈ, ਉੱਤਲ ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ, f = +25 cm
ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਲੈਂਨਜ਼ ਤੋਂ ਦੂਰੀ, υ = + 75 cm
ਵਸਤੂ ਦੀ ਲੈਂਨਜ਼ ਤੋਂ ਦੂਰੀ, u = ?
ਲੈਂਨਜ਼ ਸੂਤਰ \(\frac{1}{v}-\frac{1}{u}=\frac{1}{f}\) ਤੋਂ
\(\frac{1}{u}=\frac{1}{v}-\frac{1}{f}\)
= \(\frac{1}{75}-\frac{1}{25}\)
= \(\frac{1-3}{75}\)
= \(\frac{-2}{75}\)
⇒ u = \(\frac{-75}{2}\)
∴ u = – 37.5 cm
ਵਸਤੂ ਲੈੱਨਜ਼ ਦੇ ਖੱਬੇ ਪਾਸੇ 37.5 cm ਦੀ ਦੂਰੀ ‘ਤੇ ਸਥਿਤ ਹੈ ।
∴ ਪ੍ਰਤਿਬਿੰਬ ਲੈੱਨਜ਼ ਦੇ ਦੂਜੇ ਪਾਸੇ ਬਣਦਾ ਹੈ ਇਸ ਲਈ ਇਹ ਵਾਸਤਵਿਕ ਅਤੇ ਉਲਟਾ ਹੋਵੇਗਾ ।
ਪ੍ਰਸ਼ਨ 13.
ਹਵਾ ਦੇ ਸਾਪੇਖ ਸੰਘਣਾ ਫਟ ਕੱਚ ਦਾ ਅਪਵਰਤਨ ਅੰਕ 1.65 ਅਤੇ ਅਲਕੋਹਲ ਦੇ ਲਈ ਇਹ 1.36 ਹੈ । ਅਲਕੋਹਲ ਦੇ ਸਾਪੇਖ ਫਲਿੰਟ ਕੱਚ ਦਾ ਅਪਵਰਤਨ ਅੰਕ ਕੀ ਹੋਵੇਗਾ ?
ਹੱਲ :
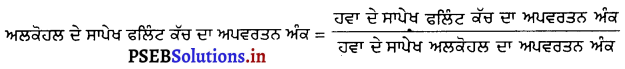
= \(\frac{1.65}{1.36}\)
= \(\frac{165}{136}\)
= 1.21
ਪ੍ਰਸ਼ਨ 14.
ਇੱਕ ਵਸਤੂ 20 ਸਮ ਫੋਕਸ ਦੂਰੀ ਵਾਲੇ ਇੱਕ ਅਵਤਲ ਲੈੱਨਜ਼ ਦੇ ਸਾਹਮਣੇ 40 ਸਮ ਦੀ ਦੂਰੀ ‘ਤੇ ਰੱਖੀ ਗਈ ਹੈ । ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਸਥਿਤੀ ਅਤੇ ਲੈੱਨਜ਼ ਦਾ ਵੱਡਦਰਸ਼ਨ ਪਤਾ ਕਰੋ !
ਹੱਲ :
ਅਵਤਲ ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ (f) = -20 ਸਮ
ਵਸਤੂ ਦੀ ਲੈਂਨਜ਼ ਤੋਂ ਦੂਰੀ (u) = -40 ਸਮ
ਤਿਬਿੰਬ ਦੀ ਲੈਂਜ਼ ਤੋਂ ਦੂਰੀ (ਸਥਿਤੀ) (υ) = ?

ਪ੍ਰਤਿਬਿੰਬ ਆਕਾਰ ਵਿਚ ਛੋਟਾ ਹੋਵੇਗਾ ।

ਪ੍ਰਸ਼ਨ 15.
ਕਿਸੇ ਮਾਧਿਅਮ ਵਿੱਚ ਪ੍ਰਕਾਸ਼ ਦਾ ਵੇਗ 2 × 108m/s ਹੈ । ਇੱਕ ਆਪਾਤੀ ਕਿਰਨ ਇਸ ਦੇ ਸੰਘਣੇ ਪਾਸੇ ਨਾਲ 30° ਦਾ ਕੋਣ ਬਣਾਉਂਦੀ ਹੈ | ਅਪਵਰਤਨ ਕੋਣ ਪਤਾ ਕਰੋ ਨਿਰਵਾਯੂ ਵਿੱਚ ਪ੍ਰਕਾਸ਼ ਦਾ ਵੇਗ = 3 × 108 m/s |
ਹੱਲ :
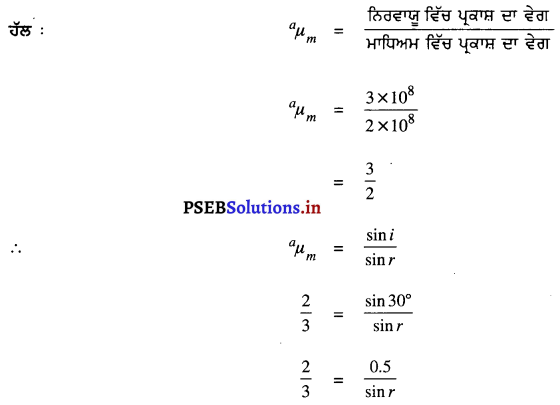
sin r = \(\frac{0.5}{\frac{2}{3}}\)
sin r = 0.7500
∴ ਅਪਵਰਤਨ ਕੋਣ, r = 48°36′
ਪ੍ਰਸ਼ਨ 16.
5 cm ਉੱਚੀ ਕੋਈ ਵਸਤੂ 10 cm ਫੋਕਸ ਦੂਰੀ ਦੇ ਅਭਿਸਾਰੀ ਲੈਂਨਜ਼ ਤੋਂ 25 cm ਦੂਰੀ ਤੇ ਰੱਖੀ ਜਾਂਦੀ ਹੈ । ਪ੍ਰਕਾਸ਼ ਕਿਰਨ ਰੇਖਾ-ਚਿੱਤਰ ਬਣਾ ਕੇ ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਸਥਿਤੀ, ਸਾਈਜ਼ ਅਤੇ ਪ੍ਰਕਿਰਤੀ ਪਤਾ ਕਰੋ ।
ਹੱਲ :
ਇੱਥੇ ਵਸਤੂ ਦੀ ਉੱਚਾਈ h1 = 5 cm
ਉੱਤਲ ਲੈੱਨਜ਼ ਤੋਂ ਵਸਤੂ ਦੀ ਦੂਰੀ u = -25 cm
ਲੈੱਨਜ਼ ਫਾਰਮੂਲਾ ਤੋਂ f = + 10 cm (ਅਭਿਸਾਰੀ ਲੈੱਨਜ਼ ਜਾਂ ਉੱਤਲ ਲੈੱਨਜ਼)
υ = ?

ਪ੍ਰਸ਼ਨ 17.
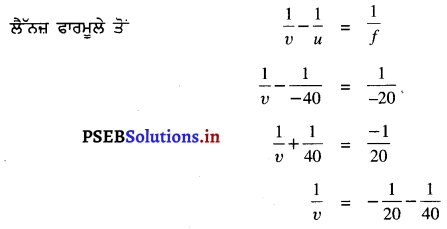
15 cm ਫੋਕਸ ਦੂਰੀ ਦਾ ਕੋਈ ਅਵਤਲ ਲੈੱਨਜ਼ ਕਿਸੇ ਵਸਤੂ ਦਾ ਲੈਂਨਜ਼ ਤੋਂ 10 cm ਦੂਰੀ ਤੇ ਪ੍ਰਤਿਬਿੰਬ ਬਣਾਉਂਦਾ ਹੈ। ਲੈੱਨਜ਼ ਤੋਂ ਕਿੰਨੀ ਦੂਰੀ ਤੇ ਵਸਤੂ ਸਥਿਤ ਹੈ ? ਚਿੱਤਰ ਵੀ ਬਣਾਓ ।
ਹੱਲ :
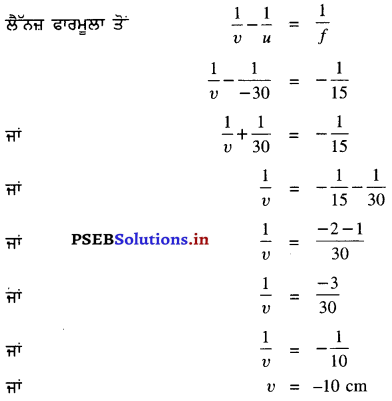
ਇੱਥੇ f = -15 cm, υ = -10 cm, u = ?
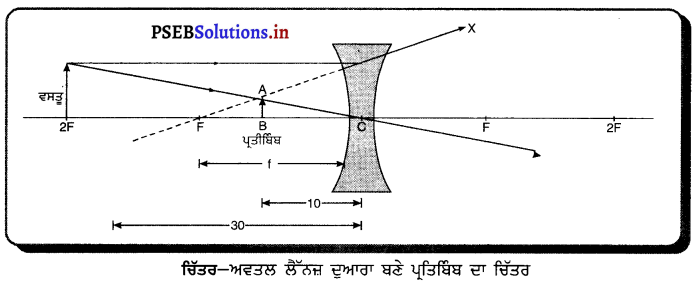
= \(\frac{-1}{10}+\frac{1}{15}\)
\(\frac{-3+2}{30}\)
= \(\frac{-1}{30}\)
u = -30 cm
ਵਸਤੂ ਨੂੰ ਅਵਤਲ ਲੈੱਨਜ਼ ਤੋਂ 30 cm ਦੀ ਦੂਰੀ ਤੇ ਰੱਖਣਾ ਚਾਹੀਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 18.
ਇੱਕ ਵਸਤੂ ਨੂੰ 15 cm ਫੋਕਸ-ਦੂਰੀ ਦੇ ਇੱਕ ਅਵਤਲ ਲੈੱਨਜ਼ ਤੋਂ 30 cm ‘ਤੇ ਰੱਖਿਆ ਜਾਂਦਾ ਹੈ। ਦੱਸੋ ਪ੍ਰਤਿਬਿੰਬ ਦਾ ਸਰੂਪ ਸਥਿਤੀ ਅਤੇ ਵੱਡਦਰਸ਼ਨ ਕੀ ਹੋਵੇਗਾ ?
ਹੱਲ :
ਅਵਤਲ ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ (f) = -15 cm (ਅਵਤਲ ਦਰਪਣ ਲਈ f ਰਿਣਾਤਮਕ ਹੁੰਦੀ ਹੈ।
ਵਸਤੁ ਦੀ ਅਵਤ ਲੈਂਨਜ਼ ਤੋਂ ਦੂਰੀ (u) = -30 cm (ਆਪਾਤੀ ਕਿਰਨ ਦੇ ਉਲਟ ਮਾਪੀ ਦੁਰੀ)
ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਅਵਤਲ ਲੈਂਨਜ਼ ਤੋਂ ਦੂਰੀ (v) = ?
υ ਦੇ ਰਿਣ ਚਿੰਨ੍ਹ ਤੋਂ ਪਤਾ ਲਗਦਾ ਹੈ ਕਿ ਪ੍ਰਤਿਬਿੰਬ ਲੈਂਨਜ਼ ਦੇ ਉਸੇ ਪਾਸੇ 10 cm ਦੀ ਦੂਰੀ ਤੇ ਬਣੇਗਾ ਜਿਸ ਪਾਸੇ ਵਸਤੂ ਰੱਖੀ ਗਈ ਹੈ । ਇਸ ਲਈ ਪ੍ਰਤਿਬਿੰਬ ਆਭਾਸੀ ਅਤੇ ਸਿੱਧਾ ਹੋਵੇਗਾ ।
ਹੁਣ ਵੱਡਦਰਸ਼ਨ (m) = \(\frac{v}{u}\)
= \(\frac{(-10)}{(-30)}\)
= \(\frac{10}{30}\)
= \(\frac{1}{3}\) = 0.33
ਕਿਉਂਕਿ ਅ ਦਾ ਮਾਨ ਧਨਾਤਮਕ ਹੈ ਇਸ ਲਈ ਪ੍ਰਤਿਬਿੰਬ ਸਿੱਧਾ ਹੈ ।
∴ |m| = \(\frac{1}{3}\) ਜੋ ਕਿ < 1 ਹੈ, ਇਸ ਲਈ ਪ੍ਰਤਿਬਿੰਬ ਸਾਈਜ਼ ਵਿੱਚ ਵਸਤੂ ਤੋਂ ਛੋਟਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 19.
7 cm ਉਚਾਈ ਦੀ ਇੱਕ ਵਸਤੂ ਨੂੰ 20 cm ਫੋਕਸ-ਦੂਰੀ ਦੇ ਇੱਕ ਉੱਤਲ ਲੈੱਨਜ਼ ਤੋਂ 40 cm ਦੀ ਦੂਰੀ ਤੇ ਰੱਖਿਆ ਜਾਂਦਾ ਹੈ । ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਸਥਿਤੀ, ਸਰੂਪ ਅਤੇ ਉੱਚਾਈ ਮਾਲੂਮ ਕਰੋ ।
ਹੱਲ :
ਵਸਤੂ ਦੀ ਉੱਚਾਈ (h) = + 7 cm
ਉੱਤਲ ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ (f)= + 20 cm
(ਉੱਤਲ ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ ਧਨਾਤਮਕ ਮੰਨੀ ਜਾਂਦੀ ਹੈ।)
ਵਸਤੂ ਦੀ ਉੱਤਲ ਲੈੱਨਜ਼ ਤੋਂ ਦੂਰੀ (u) = -40 cm
(ਆਪਾਤੀ ਕਿਰਨ ਦੇ ਉਲਟ ਦਿਸ਼ਾ ਵਿੱਚ ਮਾਪੀ ਗਈ ਦੂਰੀ)
ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਲੈਂਨਜ਼ ਤੋਂ ਦੂਰੀ (υ) = ?
∴ υ = + 40 cm
υ ਦੇ ਧਨ ਚਿੰਨ੍ਹ ਤੋਂ ਪਤਾ ਲਗਦਾ ਹੈ ਕਿ ਤਿਬਿੰਬ ਵਾਸਤਵਿਕ ਅਤੇ ਉਲਟਾ ਹੈ ਅਤੇ ਲੈੱਨਜ਼ ਦੇ ਦੂਜੇ ਪਾਸੇ 40 cm ਦੀ ਦੂਰੀ ਤੇ ਬਣਦਾ ਹੈ । ਹੁਣ m = \(\frac{v}{u}\) = \(\frac{40}{-40}\) > m = -1
\(|m|=|-1|\) = 1
ਪਰ m = \(\frac{h_{2}}{h_{1}}\)
∴ h2 = h1
ਅਰਥਾਤ ਤਿਬਿੰਬ ਦਾ ਸਾਇਜ਼ ਵਸਤੂ ਦੇ ਆਕਾਰ (ਸਾਈਜ਼) ਦੇ ਬਰਾਬਰ ਹੈ ।

ਪ੍ਰਸ਼ਨ 20.
4 cm ਉੱਚਾਈ ਦੀ ਇੱਕ ਵਸਤੂ ਨੂੰ -10 ਡਾਇਓਪਟਰ ਸ਼ਕਤੀ ਵਾਲੇ ਇਕ ਅਵਤਲ ਲੈੱਨਜ਼ ਦੇ ਸਾਹਮਣੇ 15 cm ਦੀ ਦੂਰੀ ਤੇ ਰੱਖਿਆ ਜਾਂਦਾ ਹੈ | ਪ੍ਰਤਿਬਿੰਬ ਦਾ ਸਾਈਜ਼ ਅਤੇ ਸਰੂਪ ਪਤਾ ਕਰੋ ।
ਹੱਲ :
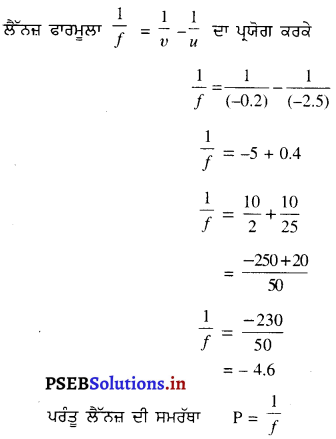
ਵਸਤੂ ਦੀ ਉੱਚਾਈ (h1) = +4 cm
ਅਵਤਲ ਲੈੱਨਜ਼ ਦੀ ਸ਼ਕਤੀ (P) = -10 ਡਾਇਪਟਰ
ਪਰ P = 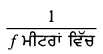
∴ f = \(\frac{1}{-10}\) × 100
= -10 cm
∴ ਅਵਤਲ ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ (f)= -10 cm
ਵਸਤੂ ਦੀ ਲੈਂਨਜ਼ ਤੋਂ ਦੂਰੀ (u) = -15 cm
ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਲੈਂਨਜ਼ ਤੋਂ ਦੂਰੀ (υ) = ?

∴ υ = -6 cm
υ ਦੇ ਰਿਣ ਚਿੰਨ੍ਹ ਤੋਂ ਪਤਾ ਲਗਦਾ ਹੈ ਕਿ ਤਿਬਿੰਬ ਆਭਾਸੀ ਅਤੇ ਸਿੱਧਾ ਹੈ ਅਤੇ ਲੈੱਨਜ਼ ਦੇ ਉਸੇ ਪਾਸੇ 6 cm ਦੀ । ਦੂਰੀ ਤੇ ਬਣਦਾ ਹੈ ।
ਹੁਣ m = \(\frac{v}{u}\)
= \(\frac{-6}{-15}\)
= \(\frac{2}{5}\)
m = \(\frac{h_{2}}{h_{1}}=\frac{2}{5}\)
∴ h2 = \(\frac{2}{5}\) × h1
= \(\frac{2}{5}\) × 4
∴ h2 = \(\frac{8}{5}\) cm
= 1.6 cm.
ਪ੍ਰਤਿਬਿੰਬ ਦਾ ਸਾਈਜ਼ 1.6 cm ਹੈ ।
ਪ੍ਰਸ਼ਨ 21.
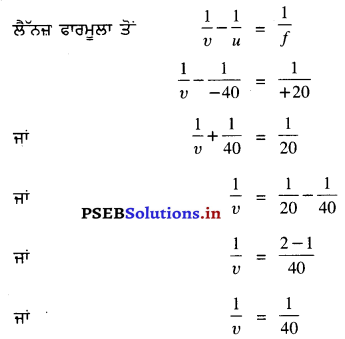
5 ਮੀਟਰ ਫੋਕਸ ਦੂਰੀ ਵਾਲੇ ਅਵਤ ਲੈਂਨਜ਼ ਦੀ ਸ਼ਕਤੀ ਪਤਾ ਕਰੋ ।
ਹੱਲ :
ਅਵਤਲ ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ (f) = -5 m
ਅਵਤਲ ਲੈੱਨਜ਼ ਦੀ ਸ਼ਕਤੀ (P) = ?
ਅਸੀਂ ਜਾਣਦੇ ਹਾਂ ਕਿ ਲੈੱਨਜ਼ ਦੀ ਸ਼ਕਤੀ (P) = \(\frac{1}{f}\) (ਮੀਟਰਾਂ ਵਿੱਚ)
= \(\frac{1}{-5}\)
= -0.2D
ਪ੍ਰਸ਼ਨ 22.
4 ਮੀਟਰ ਫੋਕਸ ਦੂਰੀ ਵਾਲੇ ਇਕ ਉੱਤਲ ਦੀ ਸ਼ਕਤੀ ਪਤਾ ਕਰੋ ।
ਹੱਲ :
ਉੱਤਲ ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ (f) = 4 ਮੀ.
ਉੱਤਲ ਲੈੱਨਜ਼ ਦੀ ਸ਼ਕਤੀ (P) = ?
ਅਸੀਂ ਜਾਣਦੇ ਹਾਂ ਕਿ ਲੈੱਨਜ਼ ਦੀ ਸ਼ਕਤੀ (P) = \(\frac{1}{f}\) (ਮੀਟਰਾਂ ਵਿੱਚ)
= \(\frac{1}{4}\)
p = 0.25D
ਪ੍ਰਸ਼ਨ 23.
5 ਮੀਟਰ ਫੋਕਸ ਦੂਰੀ ਵਾਲੇ ਇਕ ਉੱਤਲ ਲੈੱਨਜ਼ ਦੀ ਸ਼ਕਤੀ ਪਤਾ ਕਰੋ । ਲੈੱਨਜ਼ ਦੀ ਸ਼ਕਤੀ ਦੀ ਇਕਾਈ ਲਿਖੋ । (ਮਾਂਡਲ ਪੇਪਰੇ)
ਹੱਲ :
ਉੱਤਲ ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ (f) = 5 ਮੀ.
ਉੱਤਲ ਲੈੱਨਜ਼ ਦੀ ਸਕਤੀ (P) = ?
ਅਸੀਂ ਜਾਣਦੇ ਹਾਂ ਕਿ ਲੈੱਨਜ਼ ਦੀ ਸ਼ਕਤੀ (P) = \(\frac{1}{f}\)
= \(\frac{1}{5}\)
P = 0.20 D (ਡਾਇਓਪਟਰ) ਉੱਤਰ
ਬਹੁਤ ਛੋਟੇ ਉੱਤਰਾਂ ਵਾਲੇ ਪ੍ਰਸ਼ਨ (Very Short Answer Type Questions)
ਪ੍ਰਸ਼ਨ 1.
ਦਰਪਣ ਦੀ ਫੋਕਸ ਦੂਰੀ (Focal length) ਦੀ ਪਰਿਭਾਸ਼ਾ ਲਿਖੋ ।
ਉੱਤਰ-
ਫੋਕਸ ਦੂਰੀ (Focal length) – ਗੋਲਾਕਾਰ ਦਰਪਣ ਦੇ ਧਰੁਵ ਅਤੇ ਮੁੱਖ ਫੋਕਸ ਦੇ ਵਿਚਾਲੇ ਦੀ ਦੂਰੀ ਨੂੰ ਫੋਕਸ ਦੂਰੀ ਕਹਿੰਦੇ ਹਨ । S.I. ਪੱਧਤੀ ਵਿਚ ਫੋਕਸ ਦੂਰੀ ਦਾ ਮਾਤਕ ਮੀਟਰ ਹੈ ।
ਪ੍ਰਸ਼ਨ 2.
ਦਰਪਣ ਦਾ ਧਰੁਵ ਜਾਂ ਸ਼ੀਰਸ਼ ਦੀ ਪਰਿਭਾਸ਼ਾ ਲਿਖੋ ।
ਉੱਤਰ-
ਧਰੁਵ ਜਾਂ ਸ਼ੀਰਸ਼ (Pole) – ਦਰਪਣ ਦੇ ਮੱਧ ਬਿੰਦੂ ਜਾਂ ਕੇਂਦਰ ਨੂੰ ਇਸ ਦਾ ਧਰੁਵ ਜਾਂ ਸ਼ੀਰਸ਼ ਕਹਿੰਦੇ ਹਨ ।
ਪ੍ਰਸ਼ਨ 3.
ਜੇ ਕੋਈ ਵਸਤੂ ਸਮਤਲ ਦਰਪਣ ਤੋਂ 10 ਮੀਟਰ ਦੀ ਦੂਰੀ ‘ਤੇ ਹੈ, ਤਾਂ ਵਸਤੂ ਅਤੇ ਉਸ ਦੇ ਪ੍ਰਤਿਬਿੰਬ ਵਿਚ ਕਿੰਨੀ ਦੂਰੀ ਹੋਵੇਗੀ ?
ਉੱਤਰ-
ਵਸਤੁ ਅਤੇ ਪ੍ਰਤਿਬਿੰਬ ਵਿਚਕਾਰ ਦੂਰੀ = (10 + 10) ਮੀਟਰ = 20 ਮੀਟਰ ।

ਪ੍ਰਸ਼ਨ 4.
ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਦੀ ਕਿਰਨ ਸਮਤਲ ਦਰਪਣ ‘ਤੇ ਅਭਿਲੰਬ ਰੂਪ ਵਿਚ ਪੈਂਦੀ ਹੈ, ਤਾਂ ਉਸ ਦਾ ਆਪਾਤੀ ਕੋਣ ਅਤੇ ਪਰਿਵਰਤਿਤ ਕੋਣ ਕਿੰਨੀ-ਕਿੰਨੀ ਡਿਗਰੀ ਦੇ ਹੁੰਦੇ ਹਨ ?
ਉੱਤਰ-
ਆਪਤਨ ਕੋਣ (∠i) = 0
ਪਰਾਵਰਤਨ ਕੋਣ (∠r) = 0
ਪ੍ਰਸ਼ਨ 5.
ਊਰਜਾ ਦੇ ਕੋਈ ਤਿੰਨ ਰੂਪਾਂ ਦੇ ਨਾਂ ਲਿਖੋ ।
ਉੱਤਰ-
- ਪ੍ਰਕਾਸ਼ ਊਰਜਾ
- ਤਾਪ ਊਰਜਾ
- ਧੁਨੀ ਊਰਜਾ ।
ਪ੍ਰਸ਼ਨ 6.
ਪ੍ਰਕਾਸ਼ ਉਰਜਾ ਦੀ ਪ੍ਰਕਿਰਤੀ ਕੀ ਹੈ ?
ਉੱਤਰ-
ਬਿਜਲ-ਚੁੰਬਕੀ ਤਰੰਗਾਂ ।
ਪ੍ਰਸ਼ਨ 7.
ਹਵਾ ਵਿਚ ਪ੍ਰਕਾਸ਼ ਦੀ ਚਾਲ ਕਿੰਨੀ ਹੈ ?
ਉੱਤਰ-
3 × 108 m/s ।
ਪ੍ਰਸ਼ਨ 8.
ਪ੍ਰਕਾਸ਼ ਦੇ ਇੱਕ ਮੁੱਖ ਪ੍ਰਕਿਰਤਿਕ ਸੋਮੇ ਦਾ ਨਾਂ ਦੱਸੋ ।
ਉੱਤਰ-
ਸੂਰਜ ।
ਪ੍ਰਸ਼ਨ 9.
ਮਨੁੱਖ ਦੁਆਰਾ ਬਣਾਏ ਦੋ ਪ੍ਰਕਾਸ਼ ਸੋਮਿਆਂ ਦੇ ਨਾਂ ਦੱਸੋ ।
ਉੱਤਰ-
- ਮੋਮਬੱਤੀ
- ਬਿਜਲੀ ਲੈਂਪ ।
ਪ੍ਰਸ਼ਨ 10.
ਗੋਲਾਕਾਰ ਦਰਪਣ ਦੀਆਂ ਦੋ ਕਿਸਮਾਂ ਦੇ ਨਾਂ ਦੱਸੋ ।
ਉੱਤਰ-
- ਅਵਤਲ ਦਰਪਣ ਅਤੇ
- ਉੱਤਲ ਦਰਪਣ ।

ਪ੍ਰਸ਼ਨ 11.
ਆਪਨ ਕੋਣ ਕੀ ਹੁੰਦਾ ਹੈ ?
ਉੱਤਰ-
ਆਪਤਨ ਕੋਣ – ਆਪਾਤੀ ਕਿਰਨ ਅਤੇ ਅਭਿਲੰਬ ਦੇ ਵਿਚਾਲੇ ਬਣੇ ਕੋਣ ਨੂੰ ਆਪਤਨ ਕੋਣ (∠i) ਕਹਿੰਦੇ ਹਨ ।
ਪ੍ਰਸ਼ਨ 12.
ਪਰਾਵਰਤਨ ਕੋਣ ਕੀ ਹੁੰਦਾ ਹੈ ?
ਉੱਤਰ-
ਪਰਾਵਰਤਨ ਕੋਣ-ਪਰਾਵਰਤਿਤ ਕਰਨ ਅਤੇ ਅਭਿਲੰਬ ਦੇ ਵਿਚਾਲੇ ਆਪਨ ਬਿੰਦੂ ‘ਤੇ ਬਣੇ ਕੋਣ ਨੂੰ ਪਰਾਵਰਤਨ ਕੋਣ (∠r) ਕਹਿੰਦੇ ਹਨ ।
ਪ੍ਰਸ਼ਨ 13.
ਗੋਲਾਕਾਰ ਦਰਪਣ ਦੀ ਪਰਿਭਾਸ਼ਾ ਦਿਓ ।
ਉੱਤਰ-
ਗੋਲਾਕਾਰ ਦਰਪਣ (Spherical Mirror) – ਜੇਕਰ ਦਰਪਣ ਕਿਸੇ ਖੋਖਲੇ ਗੋਲੇ ਦਾ ਹਿੱਸਾ ਹੈ ਜਿਸ ਦੀ ਇੱਕ ਸਤਹਿ ਪਾਲਸ਼ ਕੀਤੀ ਹੋਈ ਅਤੇ ਦੂਜੀ ਸਤਹਿ ਪਰਾਵਰਤਕ ਹੋਵੇ ਤਾਂ ਅਜਿਹਾ ਦਰਪਣ ਗੋਲਾਕਾਰ ਦਰਪਣ ਕਹਾਉਂਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 14.
ਸਮਤਲ ਦਰਪਣ ਵਿਚ ਕਿਸ ਪ੍ਰਕਿਰਤੀ ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਬਣਦਾ ਹੈ ?
ਉੱਤਰ-
ਆਭਾਸੀ, ਸਿੱਧਾ ਅਤੇ ਸਮਾਨ ਆਕਾਰ (ਸਾਇਜ਼) ਦਾ ।
ਪ੍ਰਸ਼ਨ 15.
ਕਿਸ ਗੋਲਾਕਾਰ ਦਰਪਣ ਦੀ ਫੋਕਸ ਦੂਰੀ ਧਨਾਤਮਕ ਮੰਨੀ ਜਾਂਦੀ ਹੈ ?
ਉੱਤਰ-
ਉੱਤਲ ਦਰਪਣ ਦੀ ।

ਪ੍ਰਸ਼ਨ 16.
ਦਰਪਣ ਫਾਰਮੂਲਾ ਲਿਖੋ ।
ਜਾਂ
ਕਿਸੇ ਗੋਲਾਕਾਰ ਦਰਪਣ ਤੋਂ ਪਰਾਵਰਤਨ ਲਈ ਫਾਰਮੂਲਾ ਲਿਖੋ ।
ਉੱਤਰ-
\(\frac{1}{u}+\frac{1}{v}=\frac{1}{f}\)
ਪ੍ਰਸ਼ਨ 17.
ਅਵਤਲ ਦਰਪਣ ਦੀ ਕਿਸ ਸਤਹਿ ਤੋਂ ਪਰਾਵਰਤਨ ਹੁੰਦਾ ਹੈ ?
ਉੱਤਰ-
ਉਹ ਅੰਦਰਲੀ ਸਤਹਿ ਜੋ ਦਰਪਣ ਦੇ ਵਕ੍ਰਤਾ ਕੇਂਦਰ ਵੱਲ ਹੁੰਦੀ ਹੈ ।
ਪ੍ਰਸ਼ਨ 18.
ਕਿਸ ਗੋਲਾਕਾਰ ਦਰਪਣ ਵਿਚ ਹਮੇਸ਼ਾ ਹੀ ਪ੍ਰਤਿਬਿੰਬ ਆਭਾਸੀ, ਸਿੱਧਾ ਅਤੇ ਛੋਟਾ ਬਣਦਾ ਹੈ ?
ਉੱਤਰ-
ਉੱਤਲ ਦਰਪਣ ਵਿੱਚ ।
ਪ੍ਰਸ਼ਨ 19.
ਗੋਲਾਕਾਰ ਦਰਪਣ ਦੇ ਵਕ੍ਰਤਾ ਅਰਧ-ਵਿਆਸ ਅਤੇ ਫੋਕਸ ਦੂਰੀ ਵਿਚ ਕੀ ਸੰਬੰਧ ਹੈ ?
ਉੱਤਰ-
f = \(\frac{\mathrm{R}}{2}\)
ਪ੍ਰਸ਼ਨ 20.
ਜਿਸ ਦਰਪਣ ਦੀ ਫੋਕਸ ਦੂਰੀ-15cm ਹੋਵੇ ਉਸਦੀ ਪ੍ਰਕਿਰਤੀ ਕਿਹੋ ਜਿਹੀ ਹੋਵੇਗੀ ?
ਉੱਤਰ-
ਇਹ ਦਰਪਣ ਅਵਤਲ ਹੋਵੇਗਾ ।

ਪ੍ਰਸ਼ਨ 21.
ਇੱਕ ਦਰਪਣ ਦਾ ਵੱਡਦਰਸ਼ਨ 0.4 ਹੈ । ਇਹ ਦਰਪਣ ਕਿਸ ਤਰ੍ਹਾਂ ਦਾ ਹੈ ? ਇਸਦਾ ਪ੍ਰਤਿਬਿੰਬ ਕਿਹੋ ਜਿਹਾ ਹੋਵੇਗਾ ?
ਉੱਤਰ-
ਇਹ ਦਰਪਣ ਉੱਤਲ ਹੋਵੇਗਾ ਕਿਉਂਕਿ ਇਸ ਦਾ ਵੱਡਦਰਸ਼ਨ ਧਨਾਤਮਕ ਹੈ ਅਤੇ ਇਹ 1 ਤੋਂ ਘੱਟ ਹੈ ਪ੍ਰਤਿਬਿੰਬ ਛੋਟਾ, ਸਿੱਧਾ ਅਤੇ ਆਭਾਸੀ ਹੋਵੇਗਾ ।
ਪ੍ਰਸ਼ਨ 22.
ਵੱਡਦਰਸ਼ਨ ਦੀ ਪਰਿਭਾਸ਼ਾ ਦਿਓ । ਇਸ ਦੀ ਇਕਾਈ ਕੀ ਹੈ ?
ਉੱਤਰ-
ਵੱਡਦਰਸ਼ਨ (Magnification) = ਪ੍ਰਤਿਬਿੰਬ ਦੇ ਆਕਾਰ ਜਾਂ ਸਾਇਜ਼ ਅਤੇ ਵਸਤੂ ਦੇ ਆਕਾਰ ਜਾਂ ਸਾਇਜ਼ ਦੇ ਅਨੁਪਾਤ ਨੂੰ ਵੱਡਦਰਸ਼ਨ ਆਖਦੇ ਹਨ ।

ਵੱਡਦਰਸ਼ਨ (m) ਦੀ ਕੋਈ ਇਕਾਈ ਨਹੀਂ ਹੁੰਦੀ ਕਿਉਂਕਿ ਇਹ ਦੋਨੋਂ ਇੱਕੋ ਜਿਹੀਆਂ ਰਾਸ਼ੀਆਂ ਦਾ ਅਨੁਪਾਤ ਹੈ ।
ਪ੍ਰਸ਼ਨ 23.
ਕਿਸ ਦਰਪਣ ਨੂੰ ਅਭਿਸਾਰੀ ਅਤੇ ਕਿਸ ਦਰਪਣ ਨੂੰ ਅਪਸਾਰੀ ਕਿਹਾ ਜਾਂਦਾ ਹੈ ?
ਉੱਤਰ-
ਅਭਿਸਾਰੀ ਦਰਪਣ – ਅਵਤਲ ਦਰਪਣ
ਅਪਸਾਰੀ ਦਰਪਣ – ਉੱਤਲ ਦਰਪਣ ।
ਪ੍ਰਸ਼ਨ 24.
ਸਰਚ ਲਾਈਟ ਲਈ ਕਿਸ ਦਰਪਣ ਦਾ ਪ੍ਰਯੋਗ ਕੀਤਾ ਜਾਂਦਾ ਹੈ ?
ਉੱਤਰ-
ਅਵਤਲੇ ਦਰਪਣ ਦਾ ।
ਪ੍ਰਸ਼ਨ 25.
ਕਿਸ ਦਰਪਣ ਨੂੰ ਆਪਣੇ ਤੋਂ ਦੂਰ ਲਿਜਾਣ ਨਾਲ ਦਰਪਣ ਦਾ ਦ੍ਰਿਸ਼ਟੀ ਖੇਤਰ ਵੱਧ ਜਾਂਦਾ ਹੈ ?
ਉੱਤਰ-
ਉੱਤਲ ਦਰਪਣ ।

ਪ੍ਰਸ਼ਨ 26.
ਸਮਤਲ ਦਰਪਣ ਦੀ ਕਿੰਨੀ ਫੋਕਸ ਦੂਰੀ ਹੁੰਦੀ ਹੈ ?
ਉੱਤਰ-
ਅਨੰਤ ।
ਪ੍ਰਸ਼ਨ 27.
ਕਿਸ ਦਰਪਣ ਦੀ ਵੱਡਦਰਸ਼ਨ ਸਮਰੱਥਾ 1 ਹੁੰਦੀ ਹੈ ?
ਉੱਤਰ-
ਸਮਤਲ ਦਰਪਣ ਦੀ ।
ਪ੍ਰਸ਼ਨ 28.
ਸਮਤਲ ਦਰਪਣ ਵਿਚ ਬਣ ਰਹੇ ਪ੍ਰਤਿਬਿੰਬ ਲਈ ਵਸਤੂ ਦੂਰੀ ਅਤੇ ਪ੍ਰਤਿਬਿੰਬ ਦੂਰੀ ਵਿਚਕਾਰ ਸੰਬੰਧ ਲਿਖੋ ।
ਉੱਤਰ-
u = -v
ਪ੍ਰਸ਼ਨ 29.
ਵਾਸਤਵਿਕ ਪ੍ਰਤਿਬਿੰਬ ਤੋਂ ਕੀ ਭਾਵ ਹੈ ?
ਉੱਤਰ-
ਵਾਸਤਵਿਕ ਪ੍ਰਤਿਬਿੰਬ – ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਦੀਆਂ ਕਿਰਨਾਂ ਪਰਾਵਰਤਨ ਜਾਂ ਅਪਵਰਤਨ ਤੋਂ ਬਾਅਦ ਆਪਸ ਵਿੱਚ ਇੱਕ-ਦੂਜੇ ਨੂੰ ਇੱਕ ਬਿੰਦੂ ਤੇ ਮਿਲਦੀਆਂ ਹਨ ਤਾਂ ਉਸ ਬਿੰਦੂ ਤੇ ਵਾਸਤਵਿਕ ਪ੍ਰਤਿਬਿੰਬ ਬਣਦਾ ਹੈ । ਇਹ ਤਿਬਿੰਬ ਹਮੇਸ਼ਾ ਉਲਟਾ ਬਣਦਾ ਹੈ ਅਤੇ ਪਰਦੇ ਤੇ ਲਿਆਇਆ ਜਾ ਸਕਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 30.
ਅਵਤਲ ਦਰਪਣ ਲਈ ਜਦੋਂ ਵਸਤੂ ਅਨੰਤ ਤੇ C ਦੇ ਵਿਚਕਾਰ ਹੋਵੇ ਤਾਂ ਪ੍ਰਤਿਬਿੰਬ ਦੀ ਸਥਿਤੀ ਦੱਸੋ ।
ਉੱਤਰ-
ਜਦੋਂ ਵਸਤੂ ਅਨੰਤ ਅਤੇ cਦੇ ਵਿਚਕਾਰ ਹੋਵੇ ਤਾਂ ਅਵਤਲ ਦਰਪਣ ਵਿਚ ਪ੍ਰਤਿਬੰਬ ਅਵਤਲ ਦਰਪਣ ਦੇ ਫੋਕਸ ਅਤੇ ਵਕੁਤਾ ਕੇਂਦਰ ਦੇ ਵਿਚਕਾਰ ਬਣਦਾ ਹੈ ।

ਪ੍ਰਸ਼ਨ 31.
ਇਕ ਗੋਲਾਕਾਰ ਦਰਪਣ ਦਾ ਵਕਰਤਾ ਅਰਧ ਵਿਆਸ 24 cm ਹੈ । ਇਸ ਦੀ ਫੋਕਸ ਦੂਰੀ ਕਿੰਨੀ ਹੋਵੇਗੀ ?
ਉੱਤਰ-
ਦਿੱਤਾ ਹੈ, ਵੜਾ ਅਰਧ ਵਿਆਸ R = 24 cm
∵ ਫੋਕਸ ਦੂਰੀ f = \(\frac{R}{2}=\frac{24}{2}\)
= 12 cm
ਪ੍ਰਸ਼ਨ 32.
ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਦੀ ਕਿਰਨ ਸੰਘਣੇ ਮਾਧਿਅਮ ਤੋਂ ਵਿਰਲੇ ਮਾਧਿਅਮ ਵਿਚ ਦਾਖ਼ਲ ਹੁੰਦੀ ਹੈ ਤਾਂ ਅਭਿਲੰਬ ਦੇ ਕਿਸ ਪਾਸੇ ਝੁਕਦੀ ਹੈ ?
ਉੱਤਰ-
ਅਜਿਹੀ ਪ੍ਰਕਾਸ਼ ਦੀ ਕਿਰਨ ਅਭਿਲੰਬ ਤੋਂ ਦੂਰ ਵੱਲ ਝੁਕਦੀ ਹੈ ।
ਪ੍ਰਸ਼ਨ 33.
ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਦੀ ਕਿਰਨ ਵਿਰਲੇ ਮਾਧਿਅਮ ਤੋਂ ਸੰਘਣੇ ਮਾਧਿਅਮ ਵਿਚ ਜਾਂਦੀ ਹੈ, ਤਾਂ ਅਭਿਲੰਬ ਦੇ ਕਿਸ ਪਾਸੇ ਝੁਕਦੀ ਹੈ ?
ਉੱਤਰ-
ਅਭਿਲੰਬ ਵੱਲ ।
ਪ੍ਰਸ਼ਨ 34.
ਜਦੋਂ ਪ੍ਰਕਾਸ਼ ਵਿਰਲੇ ਮਾਧਿਅਮ ਤੋਂ ਸੰਘਣੇ ਮਾਧਿਅਮ ਵਿਚ ਜਾਂਦਾ ਹੈ, ਤਾਂ ਅਪਵਰਤਨ ਕੋਣ ਅਤੇ ਆਪਤਨ ਕੋਣ ਵਿਚੋਂ ਕਿਹੜਾ ਵੱਡਾ ਹੁੰਦਾ ਹੈ ?
ਉੱਤਰ-
ਆਪਤਨ ਕੋਣ ।
ਪ੍ਰਸ਼ਨ 35.
ਉੱਤਲ ਲੈੱਨਜ਼ ਦੁਆਰਾ ਦੂਰ ਸਥਿਤ ਵਸਤੂ ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਕਿਹੋ ਜਿਹਾ ਬਣਦਾ ਹੈ ?
ਉੱਤਰ-
ਵਾਸਤਵਿਕ, ਉਲਟਾ ਅਤੇ ਛੋਟਾ ।

ਪ੍ਰਸ਼ਨ 36.
ਕਿਸੇ ਛਪੇ ਹੋਏ ਕਾਗਜ਼ ਤੇ ਅਭਿਸਾਰੀ ਲੈਂਨਜ਼ ਰੱਖਣ ਨਾਲ ਅੱਖਰ ਕਿਹੋ ਜਿਹੇ ਨਜ਼ਰ ਆਉਂਦੇ ਹਨ ?
ਉੱਤਰ-
ਸਿੱਧੇ ਅਤੇ ਵੱਡੇ ।
ਪ੍ਰਸ਼ਨ 37.
ਪਾਣੀ ਵਿੱਚ ਰੱਖਿਆ ਸਿੱਕਾ ਉੱਠਿਆ ਹੋਇਆ ਕਿਉਂ ਦਿਖਾਈ ਦਿੰਦਾ ਹੈ ?
ਉੱਤਰ-
ਪ੍ਰਕਾਸ਼ ਅਪਵਰਤਨ ਕਾਰਨ ।
ਪ੍ਰਸ਼ਨ 38.
ਮੁੱਖ ਫੋਕਸ ਅਤੇ ਪ੍ਰਕਾਸ਼ੀ ਕੇਂਦਰ ਦੇ ਵਿਚਕਾਰ ਦੀ ਦੂਰੀ ਨੂੰ ਕੀ ਕਹਿੰਦੇ ਹਨ ?
ਉੱਤਰ-
ਫੋਕਸ ਦੂਰੀ ।
ਪ੍ਰਸ਼ਨ 39.
ਕਿਸ ਸਥਿਤੀ ਵਿਚ ਪ੍ਰਤਿਬਿੰਬ ਵਸਤੂ ਦੇ ਸਾਇਜ਼ ਦੇ ਬਰਾਬਰ ਹੁੰਦਾ ਹੈ ?
ਉੱਤਰ-
ਜਦੋਂ ਵਸਤੂ 2F ਤੇ ਹੋਵੇ ।
ਪ੍ਰਸ਼ਨ 40.
ਉੱਤਲ ਲੈਂਨਜ਼ ਨਾਲ ਆਭਾਸੀ ਅਤੇ ਵੱਡਾ ਪ੍ਰਤਿਬਿੰਬ ਕਦੋਂ ਬਣਦਾ ਹੈ ?
ਉੱਤਰ-
ਜਦੋਂ ਵਸਤੂ ਮੁੱਖ ਫੋਕਸ ਅਤੇ ਪ੍ਰਕਾਸ਼ੀ ਕੇਂਦਰ ਦੇ ਵਿਚਾਲੇ ਹੋਵੇ ।

ਪ੍ਰਸ਼ਨ 41.
ਲੈੱਨਜ਼ ਕਿਸ ਨੂੰ ਕਹਿੰਦੇ ਹਨ ? ਇਹ ਕਿੰਨੀ ਤਰ੍ਹਾਂ ਦੇ ਹੁੰਦੇ ਹਨ ?
ਉੱਤਰ-
ਲੈਂਜ਼ – ਇਕ ਅਜਿਹਾ ਪਾਰਦਰਸ਼ੀ ਮਾਧਿਅਮ ਜੋ ਦੋ ਵੱਖਰੀਆਂ ਗੋਲ ਸੜਾਵਾਂ ਨਾਲ ਘਿਰਿਆ ਹੁੰਦਾ ਹੈ । ਇਹ ਦੋ ਤਰ੍ਹਾਂ ਦੇ ਹੁੰਦੇ ਹਨ-
- ਉੱਤਲ ਲੈੱਨਜ਼
- ਅਵਤਲ ਲੈੱਨਜ਼ ।
ਪ੍ਰਸ਼ਨ 42.
ਲੈੱਨਜ਼ ਦੀਆਂ ਦੂਰੀਆਂ ਕਿਸ ਬਿੰਦੂ ਤੋਂ ਮਾਪੀਆਂ ਜਾਂਦੀਆਂ ਹਨ ?
ਉੱਤਰ-
ਪ੍ਰਕਾਸ਼ੀ ਕੇਂਦਰ ਤੋਂ ।
ਪ੍ਰਸ਼ਨ 43.
ਕਿਹੜੇ ਲੈੱਨਜ਼ ਨੂੰ ਵੱਡਦਰਸ਼ੀ ਲੈਂਜ਼ ਕਿਹਾ ਜਾਂਦਾ ਹੈ ?
ਉੱਤਰ-
ਉੱਤਲ ਲੈਂਨਜ਼ ਨੂੰ ।
ਪ੍ਰਸ਼ਨ 44.
ਲੈੱਨਜ਼ ਫਾਰਮੂਲਾ ਲਿਖੋ ।
ਉੱਤਰ-
\(\frac{1}{f}=\frac{1}{v}-\frac{1}{u}\)
ਪ੍ਰਸ਼ਨ 45.
ਲੈੱਨਜ਼ ਦੀ ਸ਼ਕਤੀ ਦੀ ਪਰਿਭਾਸ਼ਾ ਲਿਖੋ ।
ਉੱਤਰ-
ਕਿਸੇ ਲੈੱਨਜ਼ ਦੀ ਸ਼ਕਤੀ ਇਸਦੀ ਫੋਕਸ ਦੂਰੀ ਦੇ ਉਲਟ ਅਨੁਪਾਤੀ ਹੁੰਦੀ ਹੈ ।
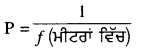
ਪ੍ਰਸ਼ਨ 46.
ਬਿਨਾਂ ਸ਼ਕਤੀ ਦੇ ਚਸ਼ਮੇ (Plane glasses) ਦੀ ਫੋਕਸ ਦੂਰੀ ਕਿੰਨੀ ਹੁੰਦੀ ਹੈ ?
ਉੱਤਰ-
P = \(\frac{1}{f}\)
f = \(\frac{1}{0}\) = ∞
ਅਨੰਤ ਫੋਕਸ ਦੂਰੀ ।

ਪ੍ਰਸ਼ਨ 47.
ਕਿਸ ਲੈਂਨਜ਼ ਨੂੰ ਅਪਸਾਰੀ ਲੈਂਨਜ਼ ਕਹਿੰਦੇ ਹਨ ?
ਉੱਤਰ-
ਅਵਤਲ ਲੈਂਨਜ਼ ਨੂੰ ।
ਪ੍ਰਸ਼ਨ 48.
ਲੈੱਨਜ਼ ਦੀ ਸ਼ਕਤੀ ਅਤੇ ਇਸ ਦੀ ਫੋਕਸ ਦੂਰੀ ਵਿੱਚ ਸੰਬੰਧ ਦੱਸੋ ।
ਉੱਤਰ-
ਪ੍ਰਸ਼ਨ 49.
ਕਿਸ ਲੈੱਨਜ਼ ਦੀ ਸ਼ਕਤੀ ਧਨ ਅਤੇ ਕਿਸ ਲੈਂਨਜ਼ ਦੀ ਸ਼ਕਤੀ ਰਿਣ ਹੁੰਦੀ ਹੈ ?
ਉੱਤਰ-
ਉੱਤਲ ਲੈੱਨਜ਼ ਦੀ ਸ਼ਕਤੀ (ਸਮਰੱਥਾ) ਧਨ ਅਤੇ ਅਵਤਲ ਲੈੱਨਜ਼ ਦੀ ਸ਼ਕਤੀ ਰਿਣ ਹੁੰਦੀ ਹੈ ।
ਪ੍ਰਸ਼ਨ 50.
ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ ਦੀ ਪਰਿਭਾਸ਼ਾ ਲਿਖੋ ।
ਉੱਤਰ-
ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ (Focal Length of Lens) – ਕਿਸੇ ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ ਉਸ ਲੈਂਨਜ਼ ਦੇ ਪ੍ਰਕਾਸ਼ੀ ਕੇਂਦਰ ਅਤੇ ਮੁੱਖ ਫੋਕਸ ਦੇ ਵਿਚਲੀ ਦੂਰੀ ਹੁੰਦੀ ਹੈ ।
ਪ੍ਰਸ਼ਨ 51.
ਅਪਵਰਤਨ-ਅੰਕ ਦੀ ਪਰਿਭਾਸ਼ਾ ਦਿਓ ।
ਉੱਤਰ-
ਅਪਵਰਤਨ-ਅੰਕ (Refractive Index) – ਨਿਰਵਾਯੂ ਵਿੱਚ ਪ੍ਰਕਾਸ਼ ਦੇ ਵੇਗ ਅਤੇ ਕਿਸੇ ਹੋਰ ਮਾਧਿਅਮ ਵਿੱਚ ਪ੍ਰਕਾਸ਼ ਦੇ ਵੇਗ ਦੇ ਅਨੁਪਾਤ ਨੂੰ ਉਸ ਮਾਧਿਅਮ ਦਾ ਨਿਰਪੇਖ ਅਪਵਰਤਨ ਅੰਕ ਕਹਿੰਦੇ ਹਨ ।

ਪ੍ਰਸ਼ਨ 52.
ਦੋ ਘੋਲਾਂ ਦੇ ਅਪਵਰਤਨ ਅੰਕ 1.36 ਅਤੇ 1.54 ਹਨ । ਇਨ੍ਹਾਂ ਘੋਲਾਂ ਵਿਚੋਂ ਕਿਹੜਾ ਘੋਲ ਵੱਧ ਸੰਘਨ ਹੈ ?
ਉੱਤਰ-
ਅਧਿਕ ਅਪਵਰਤਨ-ਅੰਕ 1.54 ਵਾਲਾ ਘੋਲ ਸੰਘਣਾ ਹੋਵੇਗਾ ।

ਪ੍ਰਸ਼ਨ 53.
ਐੱਨਜ਼ ਦੀ ਸਮਰੱਥਾ ਦਾ ਮਾਤ੍ਰਿਕ ਲਿਖੋ ।
ਉੱਤਰ-
ਡਾਈਆਪਟਰ ।
ਪ੍ਰਸ਼ਨ 54.
ਇੱਕ ਲੈੱਨਜ਼ ਦੀ ਸਮਰੱਥਾ -2.5 D ਹੈ । ਇਹ ਕਿਸ ਪ੍ਰਕਿਰਤੀ ਦਾ ਲੈਂਜ਼ ਹੈ ?
ਉੱਤਰ-
ਅਵਤਲ ਲੈੱਨਜ਼ ।
ਪ੍ਰਸ਼ਨ 55.
ਸੰਪਰਕ ਵਿੱਚ ਰੱਖੇ ਗਏ ਦੋ ਲੈੱਨਜ਼ਾਂ ਦੀ ਕੁਮਵਾਰ ਸਮਰੱਥਾ, P1 ਅਤੇ P2 ਹੈ । ਸੰਯੁਕਤ ਲੈੱਨਜ਼ ਦੀ ਸਮਰੱਥਾ ਕਿੰਨੀ ਹੋਵੇਗੀ ?
ਉੱਤਰ-
P = P1 + P2
ਪ੍ਰਸ਼ਨ 56.
ਘੜੀਸਾਜ਼ ਘੜੀ ਦੇ ਸੂਖ਼ਮ ਪੁਰਜ਼ਿਆਂ ਨੂੰ ਦੇਖਣ ਲਈ ਕਿਹੜੇ ਲੈੱਨਜ਼ ਦਾ ਪ੍ਰਯੋਗ ਕਰਦੇ ਹਨ ?
ਉੱਤਰ-
ਉੱਤਲ ਲੈੱਨਜ਼ ।
ਪ੍ਰਸ਼ਨ 57.
ਹੀਰੇ ਦਾ ਅਪਵਰਤਨ ਅੰਕ 2.42 ਹੈ ਜਦਕਿ ਕੱਚ ਦਾ ਅਪਵਰਤਨ ਅੰਕ 1.5 ਹੈ । ਦੋਨਾਂ ਵਿਚੋਂ ਕਿਹੜਾ ਵੱਧ ਸੰਘਣ ਹੈ ? ਜਿਸ ਵਿਚ ਪ੍ਰਕਾਸ਼ ਦੀ ਚਾਲ ਅਧਿਕ ਹੋਵੇਗੀ ?
ਉੱਤਰ-
ਕਿਉਂਕਿ ਹੀਰੇ ਦਾ ਅਪਵਰਤਨ ਅੰਕ ਕੱਚ ਨਾਲੋਂ ਅਧਿਕ ਹੈ ਇਸ ਲਈ ਕੱਚ ਦੀ ਤੁਲਨਾ ਵਿਚ ਹੀ ਪ੍ਰਕਾਸ਼ੀ ਸੰਘਣ ਹੈ ।
ਹੁਣ ਕਿਉਂਕਿ ਵਿਰਲੇ ਮਾਧਿਅਮ ਵਿਚ ਸੰਘਣ ਮਾਧਿਅਮ ਦੀ ਤੁਲਨਾ ਵਿਚ ਪ੍ਰਕਾਸ਼ ਦੀ ਚਾਲ ਵੱਧ ਹੁੰਦੀ ਹੈ, ਇਸ ਲਈ ਕੱਚ ਵਿਚ ਪ੍ਰਕਾਸ਼ ਦੀ ਚਾਲ ਅਧਿਕ ਹੋਵੇਗੀ ।
ਪ੍ਰਸ਼ਨ 58.
ਵਿਚਲਨ ਕੋਣ ਕੀ ਹੈ ?
ਉੱਤਰ-
ਵਿਚਲਨ ਕੋਣ – ਨਿਰਗਤ ਕਿਰਨ ਬਣਾਉਣ ਲਈ ਆਪਾਤੀ ਕਿਰਨ ਜਿਸ ਕੋਣ ਵਿਚੋਂ ਮੁੜ ਜਾਂਦੀ ਹੈ, ਉਸ ਕੋਣ ਨੂੰ ਵਿਚਲਨ ਕੋਣ ਕਹਿੰਦੇ ਹਨ ।

ਪ੍ਰਸ਼ਨ 59.
ਉੱਤਲ ਲੈੱਨਜ਼ ਦੇ ਮੁੱਖ ਫੋਕਸ ਦੀ ਪਰਿਭਾਸ਼ਾ ਲਿਖੋ ।
ਉੱਤਰ-
ਮੁੱਖ ਫੋਕਸ – ਉੱਤਲ ਲੈੱਨਜ਼ ਦਾ ਮੁੱਖ ਫੋਕਸ ਲੈੱਨਜ਼ ਦੇ ਮੁੱਖ ਧੁਰੇ ਤੇ ਉਹ ਬਿੰਦੂ ਹੈ ਜਿਸ ਤੇ ਮੁੱਖ ਧੁਰੇ ਦੇ ਸਮਾਨਾਂਤਰ ਆ ਰਹੀਆਂ ਪ੍ਰਕਾਸ਼ ਕਿਰਨਾਂ ਅਪਵਰਤਨ ਤੋਂ ਬਾਅਦ ਮਿਲਦੀਆਂ ਹਨ ।
ਪ੍ਰਸ਼ਨ 60.
ਗੋਲਾਕਾਰ ਦਰਪਣ ਦੇ ਵਤਾ ਅਰਧ-ਵਿਆਸ ਦੀ ਪਰਿਭਾਸ਼ਾ ਲਿਖੋ ।
ਉੱਤਰ-
ਵਤਾ ਅਰਧ-ਵਿਆਸ – ਗੋਲਾਕਾਰ ਦਰਪਣ ਦਾ ਅਰਧ-ਵਿਆਸ ਉਸ ਗੋਲੇ ਦਾ ਅਰਧ-ਵਿਆਸ ਹੈ ਜਿਸਦਾ ਦਰਪਣ ਹਿੱਸਾ ਹੈ । ਇਸ ਨੂੰ R ਦੁਆਰਾ ਦਰਸਾਇਆ ਜਾਂਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 61.
ਹੇਠਾਂ ਗੋਲਾਕਾਰ ਦਰਪਣ ਦੇ ਰੇਖਾ ਚਿੱਤਰ (a) ਅਤੇ (b) ਦਿੱਤੇ ਗਏ ਹਨ ? ਦਰਪਣ (a) ਅਤੇ ਦਰਪਣ (b) ਦੀ ਕਿਸਮ ਦੱਸੋ ।
ਉੱਤਰ-
(a) ਅਵਲ ਦਰਪਣ
(b) ਉੱਤਲ ਦਰਪਣ ।
ਪ੍ਰਸ਼ਨ 62.
ਹੇਠਾਂ ਦਿੱਤਾ ਚਿੱਤਰ ਕਿਸ ਪ੍ਰਕਾਸ਼ੀ ਪ੍ਰਕਿਰਿਆ ਨੂੰ ਦਰਸਾਉਂਦਾ ਹੈ ?
ਉੱਤਰ-
ਪ੍ਰਕਾਸ਼ ਅਪਵਰਤਨ ।
ਵਸਤੁਨਿਸ਼ਠ ਪ੍ਰਸ਼ਨ (Objective Type Questions)
ਪ੍ਰਸ਼ਨ 1.
ਇੱਕ ਉੱਤਲ ਲੈੱਨਜ਼ ਦੀ ਸ਼ਕਤੀ-2 ਡਾਈਆਪਟਰ ਹੈ । ਇਸਦੀ ਫੋਕਸ ਦੂਰੀ ਹੋਵੇਗੀ-
(a) 20 cm
(b) 40 cm
(c) 10 cm
(d) 50 cm.
ਉੱਤਰ-
(d) 50 cm.
ਪ੍ਰਸ਼ਨ 2.
ਵਸਤੂ ਦਾ ਆਭਾਸੀ ਅਤੇ ਬਰਾਬਰ ਆਕਾਰ ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਬਣਾਉਂਦਾ ਹੈ-
(a) ਅਵਤਲ ਦਰਪਣ
(b) ਉੱਤਲ ਦਰਪਣ
(c) ਸਮਤਲ ਦਰਪਣ
(d) ਇਹਨਾਂ ਵਿੱਚੋਂ ਕੋਈ ਵੀ ਨਹੀਂ ।
ਉੱਤਰ-
(c) ਸਮਤਲ ਦਰਪਣ ।

ਪ੍ਰਸ਼ਨ 3.
ਉੱਤਲ ਦਰਪਣ ਦੁਆਰਾ ਵਸਤੂ ਦਾ ਪ੍ਰਤਿਬਿੰਬ ਬਣਦਾ ਹੈ, ਹਮੇਸ਼ਾ
(a) ਵਾਸਤਵਿਕ, ਉਲਟਾ ਅਤੇ ਵਸਤੂ ਤੋਂ ਛੋਟਾ
(b) ਆਭਾਸੀ, ਸਿੱਧਾ ਅਤੇ ਵਸਤੂ ਤੋਂ ਛੋਟਾ
(c) ਆਭਾਸੀ, ਸਿੱਧਾ ਅਤੇ ਵਸਤੂ ਤੋਂ ਛੋਟਾ
(d) ਆਭਾਸੀ, ਸਿੱਧਾ ਅਤੇ ਵਸਤੂ ਤੋਂ ਵੱਡਾ ।
ਉੱਤਰ-
(c) ਆਭਾਸੀ, ਸਿੱਧਾ ਅਤੇ ਵਸਤੁ ਤੋਂ ਛੋਟਾ ।
ਪ੍ਰਸ਼ਨ 4.
ਮੋਟਰ ਵਾਹਨਾਂ ਵਿੱਚ ਪਿੱਛੇ ਦਾ ਦ੍ਰਿਸ਼ ਦੇਖਣ ਲਈ ਵਰਤੋਂ ਕਰਦੇ ਹਾਂ-
(a) ਅਵਤਲ ਦਰਪਣ
(b) ਸਮਤਲ ਦਰਪਣ
(c) ਉੱਤਲ ਦਰਪਣ
(d) ਕੋਈ ਵੀ ਗੋਲਾਕਾਰ ਦਰਪਣ ।
ਉੱਤਰ-
(c) ਉੱਤਲ ਦਰਪਣ ।
ਪ੍ਰਸ਼ਨ 5.
\(\frac{\sin i}{\sin r}\) ਸੰਬੰਧ ਨੂੰ ਸਭ ਤੋਂ ਪਹਿਲਾਂ ਸਥਾਪਿਤ ਕੀਤਾ-
(a) ਨਿਊਟਨ ਨੇ
(b) ਰਮਨ ਨੇ
(c) ਸਨੈਲ ਨੇ ।
(d) ਫੈਰਾਡੇ ਨੇ ।
ਉੱਤਰ-
(c) ਸਨੈਲ ਨੇ ।
ਪ੍ਰਸ਼ਨ 6.
ਕਿਸੇ ਲੈੱਨਜ਼ ਦੀ ਫੋਕਸ ਦੂਰੀ ਹੇਠ ਲਿਖਿਆਂ ਵਿੱਚੋਂ ਕਿਸ ਸੂਤਰ ਦੁਆਰਾ ਦਰਸਾਈ ਜਾਂਦੀ ਹੈ ?
(a) \(\frac{1}{f}=\frac{1}{v}+\frac{1}{u}\)
(b) \(\frac{1}{f}=\frac{1}{v}-\frac{1}{u}\)
(c) f = \(\frac{1}{v}=\frac{1}{u}\)
(d) \(\frac{1}{f}=\frac{1}{u}-\frac{1}{v}\)
ਉੱਤਰ-
(b) \(\frac{1}{f}=\frac{1}{v}-\frac{1}{u}\)
ਪ੍ਰਸ਼ਨ 7.
ਅਵਤਲ ਦਰਪਣ ਦੀ ਵਕੁਤਾ ਅਰਧ-ਵਿਆਸ R ਅਤੇ ਫੋਕਸ ਦੂਰੀ f ਵਿਚਾਲੇ ਸੰਬੰਧ ਹੁੰਦਾ ਹੈ-
(a) f = R
(b) f = \(\frac{\mathrm{R}}{2}\)
(c) R = \(\frac{f}{2}\)
(d) R = \(\frac{f}{4}\)
ਉੱਤਰ-
(b) f = \(\frac{\mathrm{R}}{2}\)

ਪ੍ਰਸ਼ਨ 8.
ਇੱਕ ਅਵਤਲ ਦਰਪਣ ਦੇ ਵਕੁਤਾ ਕੇਂਦਰ ਤੇ ਸਥਿਤ ਵਸਤੂ ਦਾ ਵਾਸਤਵਿਕ ਅਤੇ ਉਲਟਾ ਪ੍ਰਤਿਬਿੰਬ ਕਿੱਥੇ ਬਣੇਗਾ ?
(a) F ‘ਤੇ
(b) C ‘ਤੇ
(c) C ਅਤੇ F ਦੇ ਵਿਚਾਲੇ
(d) ਅਨਤ ‘ਤੇ ।
ਉੱਤਰ-
(b) C ‘ਤੇ ।
ਪ੍ਰਸ਼ਨ 9.
ਦੰਦਾਂ ਦੇ ਡਾਕਟਰ ਦੁਆਰਾ ਆਮਤੌਰ ‘ਤੇ ਵਰਤੋਂ ਵਿੱਚ ਲਿਆਉਣ ਵਾਲਾ ਦਰਪਣ-
(a) ਉੱਤਲ ਦਰਪਣ
(b) ਅਵਤਲ ਦਰਪਣ
(c) ਉੱਤਲ ਅਤੇ ਅਵਤਲ ਦਰਪਣ
(d) ਸਮਤਲ ਦਰਪਣ ।
ਉੱਤਰ-
(b) ਅਵਤਲ ਦਰਪਣ ।
ਪ੍ਰਸ਼ਨ 10.
ਵੱਡਾ ਅਤੇ ਵਾਸਤਵਿਕ ਪ੍ਰਤਿਬਿੰਬ ਪ੍ਰਾਪਤ ਕਰਨ ਲਈ ਉਪਯੋਗ ਹੋਣ ਵਾਲਾ ਦਰਪਣ-
(a) ਉੱਤਲ ਦਰਪਣ
(b) ਅਵਤਲ ਦਰਪਣ
(c) ਸਮਤਲ ਦਰਪਣ
(d) ਇਹਨਾਂ ਵਿੱਚੋਂ ਕੋਈ ਨਹੀਂ ।
ਉੱਤਰ-
(b) ਅਵਤਲ ਦਰਪਣ ।
ਖ਼ਾਲੀ ਥਾਂਵਾਂ ਭਰਨਾ
ਪ੍ਰਸ਼ਨ-ਹੇਠ ਲਿਖੀਆਂ ਖ਼ਾਲੀ ਥਾਂਵਾਂ ਭਰੋ :
(i) ਨਿਰਵਾਤ ਵਿੱਚ ਪ੍ਰਕਾਸ਼ ਦੀ ਚਾਲ ………………….. ਹੈ ।
ਉੱਤਰ-
3 × 108 ms-1
(ii) ਪ੍ਰਕਾਸ਼ …………………………. ਦੇ ਕਾਰਨ ਪਾਣੀ ਵਿੱਚ ਰੱਖਿਆ ਹੋਇਆ ਸਿੱਕਾ ਵਾਸਤਵਿਕ ਸਥਿਤੀ ਤੋਂ ਉੱਪਰ ਉੱਠਿਆ ਹੋਇਆ ਵਿਖਾਈ ਦਿੰਦਾ ਹੈ ।
ਉੱਤਰ-
ਅਪਵਰਤਨ
(iii) ਸਮਤਲ ਦਰਪਣ ਦੁਆਰਾ ਬਣਿਆ ਪ੍ਰਤਿਬਿੰਬ ਸਿੱਧਾ, ਅਭਾਸੀ ਅਤੇ …………………………. ਹੁੰਦਾ ਹੈ ।
ਉੱਤਰ-
ਵਸਤੂ ਦੇ ਬਰਾਬਰ
![]()
![]()
![]()

![]()
![]()
![]()
![]()