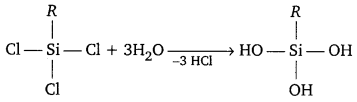Punjab State Board PSEB 11th Class Chemistry Book Solutions Chapter 11 The p-Block Elements Textbook Exercise Questions and Answers.
PSEB Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
PSEB 11th Class Chemistry Guide The p-Block Elements InText Questions and Answers
Question 1.
Discuss the pattern of variation in the oxidation states of (i) B to T1 (ii) C to Pb.
Answer:

Boron and aluminium show an oxidation state of+3 only because they do not exhibit inert pair effect due to the absence of d- or f-electrons. Elements from Ga to T1 show two oxidation states, i.e., +1 and +3. The tendency to show +1 oxidation state increases down the group due to the inability of ns2 electrons of valence shell to participate in bonding which is called inert pair effect. Therefore, Tl+ is more stable than Tl3+.

Carbon and silicon show an oxidation state of +4 only. In heavier members the tendency to show +2 oxidation state increases in the sequence Ge < Sn < Pb. It is due to the inability of ns2 electrons of valence shell to participate in bonding (inert pair effect). Ge forms stable compounds in +4 state and only few compounds in +2 state. Sn forms compounds in both the oxidation states and lead compounds in +2 state is more stable than +4 oxidation state.
![]()
Question 2.
How can you explain higher stability of BC13 as compared to T1C13?
Answer:
Boron and thallium belong to group 13 of the periodic table. In this group, the +1 oxidation state becomes more stable on moving down the , group. BCl3 is more stable than TlCl3 because the +3 oxidation state of B is more stable than the +3 oxidation state of Tl. In Tl, the +3 state is highly oxidising and it reverts back to the more stable +1 state.
Question 3.
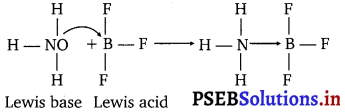
Why does boron trifluoride behave as a Lewis acid?
Answer:
BF3 being electron deficient is a strong Lewis acid. It reacts with Lewis bases easily to complete the octet around boron.
Question 4.
Consider the compounds, BCl3 and CCl4. How will they behave with water? Justify.
Answer:
Being a Lewis acid, BCI3 readily undergoes hydrolysis. As a result, Boric ‘ acid is formed.
BCl3 + 3H2O → 3HCl + B(OH)3
CCl4 completely resists hydrolysis. Carbon does not have any vacant orbital. Hence, it cannot accept electrons from water to form an intermediate. When CCl4 and water are mixed, they form separate layers.
CCl4 + H2O → No reaction.
Question 5.
Is boric acid a protic acid? Explain.
Answer:
Boric acid is not a protic acid. It is a weak monobasic acid, behaving as a Lewis acid.
B(OH)3 + 2H2O → [B (OH)4]– + H3O+
It behaves as an acid by accepting a pair of electrons from OH- ion.
Question 6.
Explain what happens when boric acid is heated.
Answer:
On heating boric acid (H3BO3) at 370 K or above, it changes to metaboric acid (HBO2). On further heating, this yields boric oxide B2O3
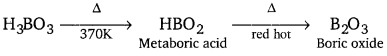
Question 7.
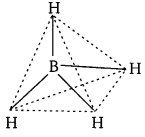
Describe the shapes of BF3 and \(\mathbf{B H}_{\mathbf{4}}^{-}\). Assign the hybridisation of boron in these species.
Answer:
(i) BF3 : As a result of its small size and high electronegativity, boron tends to form monomeric covalent halides. These halides have a planar triangular geometry. This triangular shape is formed by the overlap of three sp2 hybridised orbitals of boron with the sp-orbitals of three halogen atoms. Boron is sp2 hybridised in BF3.

(ii) \(\mathbf{B H}_{\mathbf{4}}^{-}\) : Boron-hydride ion (\(\mathbf{B H}_{\mathbf{4}}^{-}\)) is formed by the sp3 hybridisation of boron orbitals. Therefore, it is tetrahedral in structure.
![]()
Question 8.
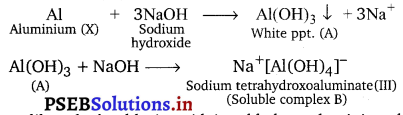
Write reactions to justify amphoteric nature of aluminium.
Answer:
A substance is called amphoteric if it displays characteristics of both acids and bases. Aluminium dissolves in both acids and bases, showing amphoteric behaviour. ..
(i) 2Al(s) + 6HCl(aq) → 2Al3+(aq) + 6Cl–(aq) + 3H2(g)
(ii) 2Al(s) + 2NaOH(aq) + 6H2O(Z) → 2Na+[Al(OH)4]–(aq) + 3H2(g)
Question 9.
What are electron deficient compounds? Are BCI3 and SiCl4 electron deficient species? Explain.
Answer:
Electron deficient compounds are those in which the octet of all the atoms is not complete i.e., all the element present in the compound do not have 8e– in their outer shell.
In trivalent state, the number of electrons around the central atom B in BCl3 is six.
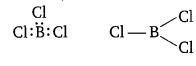
Such molecules have a tendency to accept a pair of electrons to achieve stability and hence, behave as Lewis acids.
In SiCl4, the number of electrons around the central atom Si is eight so, it is electron precise molecule.

Question 10.
Write the resonance structures of \(\mathrm{CO}_{3}^{2-}\) and \(\mathrm{HCO}_{3}^{-}\).
Answer:
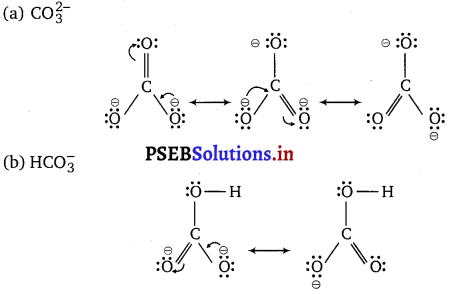
There are only two resonating structures for the bicarbonate ion.
Question 11.
What is the state of hybridisation of carbon in
(a) \(\mathrm{CO}_{3}^{2-}\)
(b) diamond
(c) graphite?
Answer:
(a) \(\mathrm{CO}_{3}^{2-}\) : C in \(\mathrm{CO}_{3}^{2-}\) is sp2 hybridised and is bonded to three oxygen atoms.
(b) Diamond : Each carbon in diamond is sp3 hybridised and is bound to four other carbon atoms.
(c) Graphite : Each carbon atom in graphite is sp2 hybridised and is bound to three other carbon atoms.
Question 12.
Explain the difference in properties of diamond and graphite on the basis of their structures.
Answer:
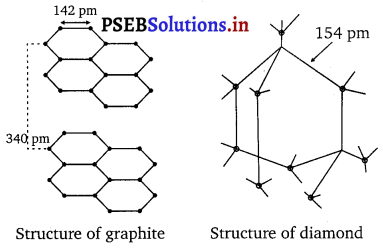
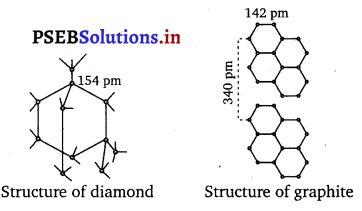
In diamond C is sp3 hybridised. Each C is tetrahedrally linked to four neighbouring carbon atoms through strong C – G sp3 – sp3 σ- bonds. The structure is highly rigid and this network extends to three dimensions. Whereas in graphite C is sp2 hybridised. Each C is linked to three other C atoms forming hexagonal rings. Thus unlike diamond, graphite has a two-dimensional sheet like (layered) structure consisting of a number of benzene rings fused together. The various sheets or layers are held together by weak van der Waals forces. Based on the above structural differences between diamond and graphite, they can be summed up as follows:
Diamond:
- It has a crystalline lattice.
- In diamond, each carbon atom is sp3 hybridised and is bonded to four other carbon atoms through a o- bond.
- It is made up of tetrahedral units.
- The C—C bond length in diamond is 154 pm.
- It has a rigid covalent bond network which is difficult to break.
- It acts as an electrical insulator.
Graphite:
- It has a layered structure.
- In graphite, each carbon atom is sp2 hybridised and is bonded to three other carbon atoms through a o- bond. The fourth electron forms a n bond.
- It has a planar geometry.
- The C—C bond length in graphite is 141.5 pm.
- It is quite soft and its layers can be separated easily.
- It is a good conductor of electricity.
![]()
Question 13.
Rationalise the given statements and give chemical reactions:
(a) Lead (II) chloride reacts with Cl2 to give PbCl4.
(b) Lead (IV) chloride is highly unstable towards heat.
(c) Lead is known not to form an iodide, Pbl4.
Answer:
(a) Lead belongs to group 14 of the periodic table. The two oxidation states displayed by this group is +2 and +4. On moving down the group the +2 oxidation state’ becomes more stable and the +4 oxidation state becomes less stable.
This is because of the inert pair effect. Hence, PbCl4 is much less stable than PbCl2. However, the formation of PbCl4 takes place when chlorine gas is bubbled through a saturated solution of PbCl2.
PbCl2(s) + Cl2(g) → PbCl4(l)
(b) On moving down group IV, the higher oxidation state becomes unstable because of the inert pair effect. Pb(IV) is highly unstable and when heated it reduces to Pb(II)
![]()
(c) Lead is known not to form Pbl4. Pb (+4) is oxidising in nature and I– is reducing in nature. A combination of Pb (IV) and iodide ion is not stable. Iodide ion is strongly reducing in nature. Pb(IV) oxidises I– to I2 and itself gets reduced to Pb (II)
PbI4 → PbI2 + I2
Question 14.
Suggest reasons why the B-F bond lengths in \(\mathbf{B F}_{3}^{-}\) (130 pm) and \(\mathbf{B F}_{4}^{-}\) (143 pm) differ.
Answer:
The B—F bond length in \(\mathbf{B F}_{3}^{-}\) is shorter than the B—F bond length in \(\mathbf{B F}_{4}^{-}\). \(\mathbf{B F}_{3}^{-}\) is an electron-deficient species. With a vacant p-orbital on boron, the fluorine and boron atoms undergo pπ—pπ back bonding to remove this deficiency. This imparts a double-bond character to the B-F bond.
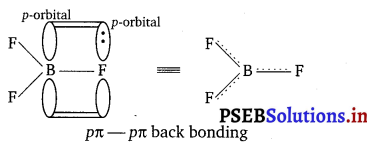
The double bond character causes the bond length to shorten in \(\mathbf{B F}_{3}^{-}\) (130 pm). However, when \(\mathbf{B F}_{3}^{-}\) coordinates with the fluoride ion, a change in hybridisation from sp2 in (\(\mathbf{B F}_{3}^{-}\)) tp sp3 (in \(\mathbf{B F}_{4}^{-}\)) occurs.
Boron now forms 4σ-bonds and the double bond character is lost. This accounts for a B—F bond length of 143 pm in \(\mathbf{B F}_{4}^{-}\) ion.
Question 15.
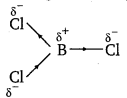
If B—Cl bond has a dipole moment, explain why BC13 molecule has zero dipole moment.
Answer:
As a result of the difference in the electronegativities of B and Cl, the B—Cl bond is polar in nature. However the BC13 molecule is non-polar. This is because BCl3 is trigonal planar in shape. It is a symmetrical molecule. Hence, the respective dipole-moments of the B-Cl bond cancel each other, thereby causing a zero-dipole moment.
Question 16.
Aluminium trifluoride is insoluble in anhydrous HF but dissolves on addition of NaF. Aluminium trifluoride precipitates out of the resulting solution when gaseous BF3 is bubbled through. Give reasons.
Answer:
Hydrogen fluoride (HF) is a covalent compound and has a very strong intermolecular hydrogen-bonding. Thus, it does not provide ions and aluminium fluoride (AlF) does not dissolve in it. Sodium fluoride (NaF) is an ionic compound and when it is added to the mixture, AlF dissolves. This is because of the availability of free F–. The reaction involved in the process is:
![]()
When boron trifluoride (BF3) is added to the solution, aluminium fluoride precipitates out of the solution. This happens because the tendency of boron to form complexes is much more than that of aluminium. Therefore, when BF3 is added to the solution, B replaces Al from the complexes according to the following reaction :
![]()
![]()
Question 17.
Suggest a reason why CO is poisonous?
Answer:
Carbon monoxide is highly-poisonous because of its ability to form a complex with haemoglobin. The CO—Hb complex is more stable than the O2—Hb complex. The former prevents Hb from binding with oxygen. Thus, a person dies because of suffocation on not receiving oxygen. It is found that the CO-Hb complex is about 300 times more stable than the O2 – Hb complex.
Question 18.
How is excessive content of CO2 responsible for global warming?
Answer:
Carbon dioxide is a very essential gas for our survival. However, an increased content of CO2 in the atmosphere posses a serious threat. An increment in the combustion of fossil fuels, decomposition of limestone, and a decrease in the number of trees had led to greater levels of carbon dioxide. Carbon dioxide has the property of trapping the heat provided by sunrays. Higher the level of carbon dioxide, higher is the amount of heat trapped. This results in an increase in the atmospheric temperature, thereby causing global warming.
Question 19.
Explain structures of diborane and boric acid.
Answer:
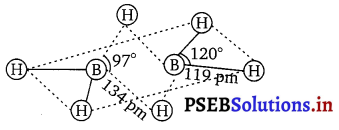
(a) Diborane : B2H6 is an electron-deficient compound. B2H6 has only
12 electrons-6e~ from 6 H atoms and 3e– each from 2 B atoms. Thus, after combining with 3 H atoms, none of the boron atoms has any electrons left. X-ray diffraction studies have shown the structure of diborane as:
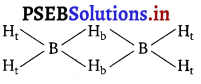
Ht = terminal hydrogen
Hb = bridging hydrogen
2 boron and 4 terminal hydrogen atoms (Ht) lie in one plane, while the other two bridging hydrogen atoms (Hb) lie in a plane perpendicular to the plane of boron atoms. Again of the two bridging hydrogen atoms, one H atom lies above the plane and the other lies below the plane. The terminal bonds are regular two-centre two-electron (2c – 2e–) bonds while the two bridging (B—H—B) bonds are three centre two electron (3c – 2e–) bonds,
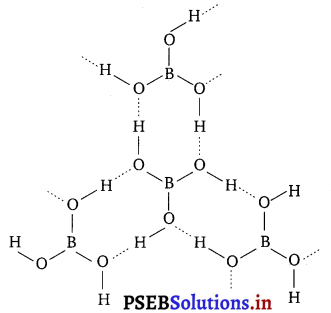
(b) Boric acid : Boric acid has a layered structure. Each planar BO3 unit is linked to one another through H atoms. The H atoms form a covalent bond with a BO3 unit, while a hydrogen bond is formed with another BO3 unit. In the given figure, the dotted lines represent hydrogen bonds.
Question 20.
What happens when
(a) Borax is heated strongly,
(b) Boric acid is added to water,
(c) Aluminium is treated with dilute NaOH,
(d) BF3 is reacted with ammonia?
Answer:
(a) When heated, borax undergoes various transitions. It first loses water molecules and swells. Then, it turns into a transparent liquid, solidifying to form a glass-like material called borax bead.
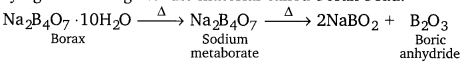
(b) When boric acid is added to water, it accepts electrons from OH- ions.
B(OH)3 +2HOH → [B(OH)4]– + H3O+
(c) A1 reacts with dilute NaOH to form sodium tetrahydroxoaluminate (III). Hydrogen gas is liberated in the process.
Al(s) + 2NaOH(aq) + 6H2O(I) → 2Na+Al(OH)4(aq) + 3H2(g)
(d) BF3 (a Lewis acid) reacts with NH3 (a Lewis base) to form an adduct. This results in a complete octet around B in BF3.
F3B + :NH3 → F3B ← NH3
![]()
Question 21.
Explain the following reactions
(a) Silicon is heated with methyl chloride at high temperature in the presence of copper.
(h) Silicon dioxide is treated with hydrogen fluoride.
(c) CO is heated with ZnO.
(d) Hydrated alumina is treated with aqueous NaOH solution.
Answer:
(a) When silicon reacts with methyl chloride in the presence of copper (catalyst) and at a temperature of about 537 K, a class of organosilicon polymers called methyl-substituted chlorosilanes (MeSiCl3, Me2SiCl2, Me3SiCl, andMe4Si) are formed.
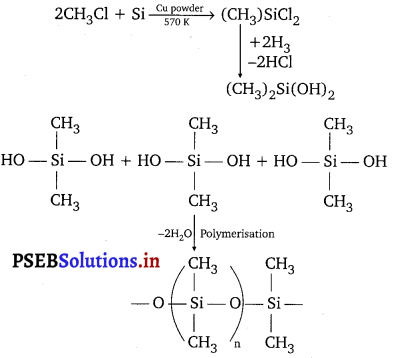
(b) When silicon dioxide (SiO2) is heated with hydrogen fluoride (HF), it forms silicon tetrafluoride (SiF4). Usually, the Si-0 bond is a strong bond and it resists any attack by halogens and most acids, even at a high temperature. However, it is attacked by HF.
SiO2 + 4HF → SiF4 + 2H2O
The SiF4 formed in this reaction can further react with HF to form hydrofluorosilicic acid.
SiF4 + 2HF → H2SiF6
(c) When CO reacts with ZnO, it reduces ZnO to Zn. CO acts as a reducing agent.
![]()
(d) When hydrated alumina is added to sodium hydroxide, the former dissolves in the latter because of the formation of sodium meta-aluminate.
Al2O3 . 2H2O + 2NaOH → 2NaAlO2 + 3H2O
Question 22.
Give reasons:
(i) Cone. HNO3 can be transported in aluminium container,
(ii) A mixture of dilute NaOH and aluminium pieces is used to open drain.
(iii) Graphite is used as lubricant.
(iv) Diamond is used as an abrasive.
(v) Aluminium alloys are used to make aircraft body.
(vi) Aluminium utensils should not be kept in water overnight.
(vii) Aluminium wire is used to make transmission cables.
Answer:
(i) Concentrated HNO3 can be stored and transported in aluminium containers as it reacts with aluminium to form a thin protective oxide layer on the aluminium surface. This oxide layer renders aluminium passive.
(ii) Sodium hydroxide and aluminium react to form sodium tetrahydroxoaluminate(III) and hydrogen gas. The pressure of the produced hydrogen gas is used to open blocked drains.
2A1 + 2NaOH + 6H2O → 2Na+ [Al(OH)4]– + 3H2
(iii) Graphite has a layered structure and different layers of graphite are bonded to each other by weak van der Waals’ forces. These layers can slide over each other. Graphite is soft and slippery. Therefore, graphite can be used as a lubricant.
(iv) In diamond, carbon is sp3 hybridised. Each carbon atom is bonded to four other carbon atoms with the help of strong covalent bonds. These covalent bonds are present throughout the surface, giving it a very rigid 3-D structure. It is very difficult to break this extended covalent bonding and for this reason, diamond is the hardest substance known. Thus, it is used as an abrasive and for cutting tools.
(v) Aluminium has a high tensile strength and is very light in weight. It can also be alloyed with various metals such as Cu, Mn, Mg, Si and Zn. It is very malleable and ductile. Therefore, it is used in making aircaft bodies.
(vi) The oxygen present in water reacts with aluminium to form a thin layer of aluminium oxide. This layer prevents aluminium from further reaction. However, when water is kept in an aluminium vessel for long period of time, some amount of aluminium oxide may dissolve in water. As aluminium ions are harmful, water should not be stored in aluminium vessels overnight.
(vii) Silver, copper, and aluminium are among the best conductors of – electricity. Silver is an expensive metal and silver wires are very expensive. Copper is quite expensive and is also very heavy. Aluminium is a very ductile metal. Thus, aluminium is used in making wires for electrical conduction.
![]()
Question 23.
Explain why is there a phenomenal decrease in ionisation enthalpy from carbon to silicon?
Answer: Electronic configuration of C is = 1s2, 2s2, 2p2
Electronic configuration of Si = 1s2, 2s22p6, 3s23p2
As we proceed from C to Si in group 14, there is a sharp increase in covalent radius from 77 pm for C to 118 pm for silicon. Due to the increase in the size of the atom, there is a sharp fall in the value of ionisation enthalpy from carbon to silicon.
Question 24.
How would you explain the lower atomic radius of Ga as compared to Al?
Answer: Electronic configuration of Al and Ga are as 13Al = 1s2 2s2 2p6 3s2 3p1 ;
31Ga = 1s22s22p63s23p63d104s24p1
The screening tendency of d-electrons is poor. Thus, On moving from Al to Ga, shielding effect of 10 d-electrons is unable to compensate increased nuclear charge. Therefore, atomic radius of Ga is smaller than that of aluminium due to effective nuclear charge.
Question 25.
What are allotropes? Sketch the structure of two allotropes of carbon namely diamond and graphite. What is the impact of structure on physical properties of two allotropes?
Answer:
The phenomenon of existence of an element in two or more forms which differ in physical properties but have almost same chemical nature is known as allotropy and the different forms of the element are known as allotropes.
Crystalline carbon occurs mainly in two allotropic forms (i) graphite and (ii) diamond. A third allotropic form of carbon called fullerene was discovered in 1985 by H.W. Kroto, E. Smalley and R.F. Curl.
In diamond, each carbon is sp3 hybridised and is linked to other four atoms tetrahedrally. There is three dimensional network of carbon atoms in diamond. In graphite, each carbon is sp2 hybridised and makes three sigma bonds with three neighbouring carbon atoms. It has layered structure and the layers are held by weak van der Waals’ forces.
Impact of the structures of diamond and graphite on physical properties of the two allotropes are as follows :
- Diamond because of its h. iness, is used as an abrasive and in making dyes while graphite is soft that it marks paper and it is used as a dry lubricant in machines.
- Diamond does not conduct electricity whereas graphite is a good conductor of electricity because of the presence of one free electron in each carbon atom.
- Diamond is transparent while graphite is opaque.
Question 26.
(a) Classify following oxides as neutral, acidic, basic or amphoteric:
CO, B2O3, SiO2, CO2, Al2O3, PbO2, Tl2O3
(b) Write suitable chemical equations to show their nature. Answer: (a) CO is neutral oxide.
B2O3, SiO2, CO2 are acidic oxides.
PbO2 Al2O3 are amphoteric oxides.
Tl2O3 is basic oxide.
(b) (i) Being acidic B2O3, SiO2 and CO2 react with alkalis to form salts.
B2O3 + CuO → CU(BO2)2
SiO2 + 2NaOH → Na2SiO3 + H2O
CO2 + H2O → H2CO3
(ii) Being amphoteric PbO2 and Al2O3 react with both acids and basses.
PbO2 + 4HCl → PbCl4 + 2H2O
PbO2 + 2NaOH → Na2PbO3 + H2O
Al2O3 + 6HCl → 2AlCl3 + 3H2O
Al2O3 + 2NaOH → 2NaAlO2 + H2O
(iii) Being basic Tl2O3 react with acid
Tl2O3 + 3H2SO4 → Tl2(SO4)3 + 3H2O
Question 27.
In some of the reactions thallium resembles aluminium, whereas in others it resembles with group I metals. Support this statement by giving some evidences.
Answer:
Thallium belongs to group 13 of the periodic table. The most common oxidation state for this group is +3. However, heavier members of this group also display the +1 oxidation state. This happens because of the inert pair effect. Aluminium displays the +3 oxidation state and alkali metals display the +1 oxidation state. Thallium displays both the oxidation states. Therefore, it resembles both aluminium and alkali metals.
Thallium, like aluminium, forms compounds such as TlCl3 and Tl2O3. It resembles alkali metals in compounds Tl20 and TlCl.
![]()
Question 28.
When metal X is treated with sodium hydroxide, a white precipitate (A) is obtained, which is soluble in excess of NaOH to give soluble complex (B). Compound (A) is soluble in dilute HC1 to form compound (C). The compound (A) when heated strongly gives (D), which is used to extract metal. Identify (X), (A), (B), (C) and (D). Write suitable equations to support their identities.
Answer:
The given metal X gives a white precipitate with sodium hydroxide and the precipitate dissolves in excess of sodium hydroxide. Hence, X must be aluminium.
The white precipitate (compound A) obtained is aluminium hydroxide. When an excess of the base is added the compound B formed is sodium tetrahydroxoaluminate (III).
Now, when dilute hydrochloric acid is added to aluminium hydroxide, aluminium chloride (compound C) is obtained.
![]()
Also, when compound A is heated strongly, it gives compound D. This compound is used to extract metal X. Aluminium metal is extracted from alumina. Hence, compound D must be alumina.
![]()
Question 29.
What do you understand by (a) inert pair effect (b) allotropy (c) catenation?
Answer:
(a) Inert pair effect : As one moves down the group, the tendency of s-block electrons to participate in chemical bonding decreases. This effect is known as inert pair effect. In case of group 13 elements, the electronic configuration is ns2 np1 and their group valency is +3. However, on moving down the group, the +1 oxidation state becomes more stable. This happens because of the poor shielding of the ns2 electrons by the d-and f-electrons. As a result of the poor shielding, the ns2 electrons are held tightly by the nucleus and so, they cannot participate in chemical bonding.
(b) Allotropy : Allotropy is the existence of an element in more than one form, having the same chemical properties but different physical properties. The various forms of an element are called allotropes. For example, carbon exists in three allotropic forms: diamond, graphite, and fullerenes.
(c) Catenation : The atoms of some elements (such as carbon) can link with one another through strong covalent bonds to form long chains or branches. This property is known as catenation. It is most common in carbon and quite significant in Si and S. *
Question 30.
A certain salt X, gives the following results :
(i) Its aqueous solution is alkaline to litmus.
(ii) It swells up to a glassy material Y on strong heating.
(iii) When cone. H2SO4 is added to a hot solution of X, white crystal of an acid Z separates out.
Write equations for all the above reactions and identify X, Y and z.
Answer:
(i) Aqueous solution of salt X is alkaline. It indicates that ‘X’ is the salt of a strong base and a weak acid.
(ii) On strong heating, the salt ‘X’ swells up to a glassy material Y. It indicates that the salt ‘X’ is borax.
(iii) Hot aqueous solution of borax on reaction with cone. H2SO4 gives crystals of orthoboric acid.
The equations for the reactions involved in the question are as follows :
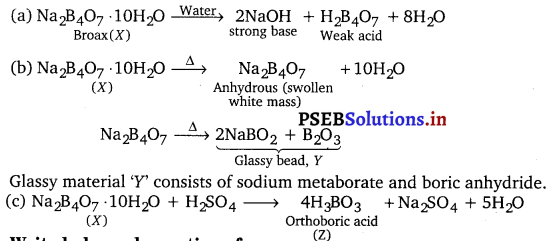
![]()
Question 31.
Write balanced equations for:
(i) BF3 + LiH
(ii) B2H6 + H2O
(iii) NaH + B2H6
(iv) im
(v) Al + NaOH
(vi) B2H6 + NH3
Answer:
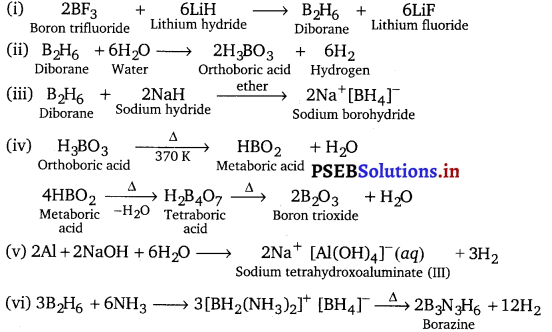
Question 32.
Give one method for industrial preparation and one for laboratory preparation of CO and CO2 each.
Answer:
Carbon monoxide : In the laboratory, CO is prepared by the dehydration of formic acid with cone. H2S04 at 373 K. The reaction involved is as follows :
![]()
CO is commercially prepared by passing steam over hot coke. The reaction involved is as follows:
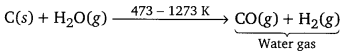
Carbon dioxide : In the laboratory, CO2 can be prepared by the action of dilute hydrochloric acid on calcium carbonate. The reaction involved is as follows:
CaCO3 + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
CO2 is commercially prepared by heating limestone. The reaction involved is as follows:
![]()
Question 33.
An aqueous solution of borax is
(a) neutral
(b) amphoteric
(c) basic
(d) acidic
Answer:
(c) Borax is a salt of a strong base (NaOH) and weak acid (H3BO3). It is, therefore, basic in nature.
Question 34.
Boric acid is polymeric due to
(a) its acidic nature
(b) the presence of hydrogen bonds
(c) its monobasic nature
(d) its geometry
Answer:
(b) Boric acid is polymeric because of the presence of hydrogen bonds, (as it has polar O—H bonds)
Question 35.
The type of hybridisation of boron in diborane is (a) sp (b) sp2 (c) sp3 (d) dsp2
Answer:
(c) Boron in diborane is sp3 hybridised.
Question 36.
Thermodynamically the most stable form of carbon is
(a) diamond
(b) graphite
(c) fullerenes
(d) coal
Answer:
(b) Graphite is thermodynamically the most stable form of carbon.
![]()
Question 37.
Elements of group 14
(a) exhibit oxidation state of +4 only
(b) exhibit oxidation state of +2 and +4
(c) formM2- and M4+ ions
(d) formM2+ and M4+ ions
Answer:
(b) The elements of group 14 have 4 valence electrons. Therefore, the oxidation state of the group is +4. However, as a result of the inert pair effect, the lower oxidation state becomes more and more stable and the higher oxidation state becomes less stable. Therefore, this group exhibits +4 and +2 oxidation states.
Question 38.
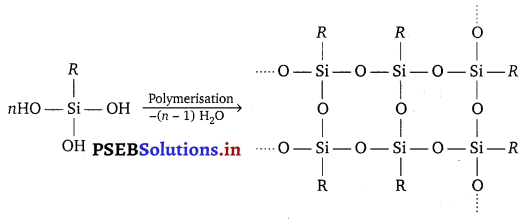
If the starting material for the manufacture of silicones is RSiCl3, write the structure of the product formed.
Answer:
Hydrolysis of alkyltrichlorosilanes followed by condensation polymerisation gives cross-linked silicones.