Punjab State Board PSEB 11th Class Physics Important Questions Chapter 11 Thermal Properties of Matter Important Questions and Answers.
PSEB 11th Class Physics Important Questions Chapter 11 Thermal Properties of Matter
Very Short Answer Type Questions
Question 1.
Is it correct to call heat as the energy in transit?
Answer:
Yes, it is perfect correct to call heat as the energy in transit because it is continuously flowing on account of temperature differences between bodies or parts of a system.
Question 2.
Why should a thermometer bulb have a small heat capacity?
Answer:
The thermometer bulb having small heat capacity will absorb less heat from the body whose temperature is to be measured. Hence, the temperature of that body will practically remain unchanged.
Question 3.
Why is a gap left between the ends of two railway lines in a railway track?
Answer:
It is done to accommodate the linear expansion of railway line during summer. If the gap is not left in summer, the lines will bend causing a threat of derailment.
Question 4.
Why water is used as an coolant in the radiator of cars?
Answer:
Because specific heat of water is very high due to this it absorbs a large amount of heat. This helps in maintaining the temperature of the engine low.
![]()
Question 5.
Black body radiation is white. Comment.
Answer:
The statement is true. A black body absorbs radiations of all wavelengths. When heated to a suitable temperature, it emits radiations of all wavelengths. Hence, a black body radiation is white.
Question 6.
White clothes are more comfortable in summer while colourful clothes are more comfortable in winter. Why?
Answer:
White clothes absorb very little heat radiation and hence they are comfortable in summer. Coloured clothes absorb almost whole of the incident radiation and keep the body warm in winter.
Question 7.
Can we boil water inside in the earth satellite?
Answer:
No, the process of transfer of heat by convection is based on the fact that a liquid becomes lighter on becoming hot and rise up. In condition of weightlessness, this is not possible. So, transfer of heat by convection is not possible in the earth satellite.
Question 8.
What is the difference between the specific heat and the molar specific heat?
Answer:
The specific heat is the heat capacity per unit mass whereas the molar specific heat is the heat capacity per mole.
Question 9.
Calorimeters are made of metals not glass. Why?
Answer:
This is because metals are good conductors of heat and have low specific heat capacity.
Question 10.
Calculate the temperature which has numeral value of Celsius and Fahrenheit scale. (NCERT Exemplar)
Answer:
Let Q be the value of temperature having same value an Celsius and Fahrenheit scale.
\(\frac{{ }^{\circ} F-32}{180}=\frac{{ }^{\circ} C}{100}\)
⇒ Let F = C = Q
⇒ \(\frac{Q-32}{180}=\frac{Q}{100}\) = Q= 40°C or -40°F
Short Answer Type Questions
Question 1.
In what ways are the gas thermometers superior to mercury thermometers?
Answer:
A gas thermometer is more superior to a mercury thermometer, as its working is independent of the nature of gas (working substance) used. As the variation of pressure (or volume) with temperature is uniform, the range, in which temperature can be measured with a gas thermometer is quite large. Further, a gas thermometer is more sensitive than mercury thermometer.
Question 2.
The difference between length of a certain brass rod and that of a steel rod is claimed to be constant at all temperatures. Is this possible?
Solution:
Yes, it is possible to describe the difference of length to remain constant. So, the change in length of each rod must be equal at all temperature. Let αb and αs be the length of the brass and the steel rod and a band as be the coefficients of linear expansion of the two metals. Let there is change in temperature be ΔT.
Then, αbLbΔT = αsLsΔT
or αbLb=αsLs => Lb/Ls=αs/αb
Hence, the lengths of the rods must be in the inverse ratio of the coefficient of linear expansion of their materials.
![]()
Question 3.
Two identical rectangular strips-one of copper and the other of steel are riveted to form a bimetallic strip. What will happen on heating?
Solution:
The coefficient of linear expansion of copper is more than steel. On heating, the expansion in copper strip is more than the steel strip. The bimetallic strip will bend with steel strip on inner (concave) side.
Question 4.
What kind of thermal conductivity and specific heat requirements would you specify for cooking utensils?
Solution:
A cooking utensil should have (i) high conductivity, so that it can conduct heat through itself and transfer it to the contents quickly, (ii) low specific heat, so that it immediately attains the temperature of the source.
Question 5.
Woollen clothes are warm in winter. Why?
Solution:
Woollen fibres enclose a large amount of air in them. Both wool and air are bad conductors of heat. The small coefficient of thermal conductivity prevents the loss of heat from our body due to conduction. So, we feel warm in woollen clothes.
Question 6.
Why rooms are provided with the ventilators near the roof?
Solution:
It is done so to remove the harmful impure air and to replace it by the cool fresh air. The air we breathe out is warm and so it is lighter. It rises upwards and can go out through the ventilator provided near the roof. The cold fresh air from outside enters the room through the doors and windows. Thus, the convection current is set up in the air.
Question 7.
Why it is much hotter above a fire than by its side?
Solution:
Heat carried away from a fire sideways mainly by radiation. Above the fire, heat is carried by both radiation and convection of air but convection carries much more heat than radiation. So, it is much hotter above a fire than by its sides.
Question 8.
How does tea in a Thermo flask remain hot for a long time?
Solution:
The air between the two walls of the Thermo flask is evacuated. This prevents heat loss due to conduction and convection. The loss of heat due to radiation is minimised by silvering the inside surface of the double wall. As the loss of heat due to the three prócesses is minimised and the tea remains hot for a long time.
![]()
Question 9.
100 g of water is supercooled to -10°C. At this point, due to some disturbance mechanised or otherwise, some of it suddenly freezes to ice. What will be the temperature of the resultant mixture and how much mass would freeze? [Sw = 1 cal/g/°C and Lwfusion =80 cal/g/°C] (NCERT Exemplar)
Answer:
Gwen, mass of water (m) = 100
Change in temperature, ΔT =0 – (-10) = 10°C
Specific heat of water (Sw) =1 cal/g/°C
Latent heat of fusion of water Lwfusion = 80 cal/g
Heat required to bring water in supercooling from —10° C to 0°C.
Q = mswΔT
=100 x 1 x 10 = 1000cal
Let m gram of ice be melted.
∴ Q = mL
or m= \(\frac{Q}{L}\) = \(\frac{1000}{80}\) =12.5g
As small mass of ice is melted, therefore the temperature of the mixture will remain 0°C.
Long Answer Type Questions
Question 1.
Show that the coefficient of volume expansion for a solid substance is three times its coefficient of linear expansion.
Solution:
Consider a solid in the form of a rectangular parallelopiped of sides a, b and c respectively so that its volume V = abc.
If the solid is heated so that its temperature rises by ΔT, then increase in its sides will be
Δa=a.αΔT, Δb=b.α.ΔT and Δc=c. α . ΔT
or a’ =a+Δa =a(1 +α ΔT)
b’=b+Δb = b(l +α ΔT)
and c’ =c + Δc=c (1 +a.ΔT)
∵ New volume, V’ = V + ΔV = a’ b’ c’ = abc (1+ α . Δ T)3
∴ Increase in volume,
ΔV=V’ -V=[abc(1+α ΔT)3 -abc]
∴ Coefficient of volume expansion,
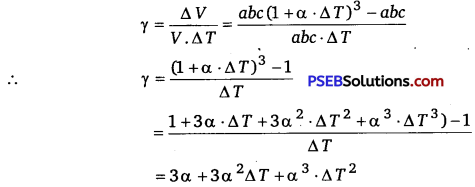
However, as a has an extremely small value for solids, hence terms containing higher powers of a may be neglected. Therefore, we obtain the relation γ =3 α i. e., coefficient of volume expansion of a solid is three times of its coefficient of linear expansion.
![]()
Question 2.
Distinguish between conduction, convection and radiation.
Solution:
| Conduction | Convection | Radiation |
| 1. It is the transfer of heat by direct physical contact. | 1. It is the transfer of heat by the motion of a fluid. | 1. It is the transfer of heat by electromagnetic waves. |
| 2. It is due to temperature differences. Heat flows from high-temperature region to low temperature region. | 2. It is due to difference in density. Heat flows from low-density region to high-density region. | 2. It occurs from all bodies at temperatures above 0 K. |
| 3. It occurs in solids through molecular collisions, without actual flow of matter. | 3. It occurs in fluids by actual flow of matter. | 3. It can take place at large distances and does not heat the intervening medium. |
| 4. It is a slow process. | 4. It is also a slow process. | 4. It propagates at the speed of light. |
| 5. It does not obey the laws of reflection and refraction. | 5. It does not obey the laws of reflection and refraction. | 5. It obeys the laws of reflection and refraction. |
