Punjab State Board PSEB 12th Class Chemistry Important Questions Chapter 10 Haloalkanes and Haloarenes Important Questions and Answers.
PSEB 12th Class Chemistry Important Questions Chapter 10 Haloalkanes and Haloarenes
Very Short Answer Type Questions
Question 1.
Out of ![]() and
and 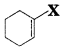 , which is an example of allylic halide?
, which is an example of allylic halide?
X
Answer:
![]() is an example of allylic halide.
is an example of allylic halide.
Question 2.
Which of the following reactions is SN1 type ?
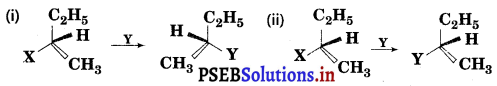
Answer:
Reaction (ii) is SN1 reaction.
Question 3.
Which one of the following compounds is more easily hydrolysed by KOH and why?
CH3CHClCH2CH3 or CH3CH2CH2CH2Cl
Answer:
Due to +1 effect of alkyl groups the 2° carbonium ion CH3—CH—CH2—CH3 derived from sec-butyl chloride is more stable than the 1° carbonium ion \(\mathrm{CH}_{3}-\mathrm{CH}_{2}-\stackrel{+}{\mathrm{C}} \mathrm{H}_{2}\) derived from n-propyl chloride. Therefore sec-butyl chloride gets hydrolysed more easily than n-propyl chloride under SN1 conditions.
![]()
Question 4.
What is known as a racemic mixture ? Give an example.
Answer:
equimolar mixture of a pair of enantiomers is called racemic mixture. A racemic mixture is optically inactive due to external compensation.
Question 5.
Consider the three types of replacement of group X by group Y as shown here.
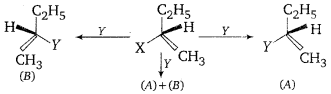
This can result in giving compound (A) or (B) or both. What is the process called if
(A) is the only compound obtained ?
(B) is the only compound obtained ?
(A) and (B) are formed in equal proportions ?
Answer:
(i) Retention
(ii) Inversion
(iii) Racemisation.
Question 6.
What is an asymmetric carbon?
Answer:
A carbon which is attached to four different atoms/groups is called asymmetric carbon. For example, the carbon atom in BrCHClI.
![]()
Question 7.
What is plane polarized light?
Answer:
A beam of light which has vibration in only one plane is called plane polarized light.
Question 8.
Why iodoform has appreciable antiseptic property ?
Answer:
Iodoform liberate I2 when it comes in contact with skin. Antiseptic property of iodine is due to the liberation of I2 not because of iodoform itself.
![]() (responsible for antiseptic property)
(responsible for antiseptic property)
Question 9.
How does the ordinary light differ from the plane polarized light?
Answer:
Ordinary light has oscillations in all the directions perpendicular to the path of propagation whereas plane polarised light has all oscillations in the same plane.
![]()
Question 10.
What do you .understand by the term optical activity of compounds?
Answer:
The property of certain compounds to rotate the plane of polarzed light in a characteristic way when it is passed through their solutions is called optical activity of compounds.
Short Answer Type Questions
Question 1.
Give reasons for the following:
(i) Ethyl iodide undergoes SN2 reaction faster than ethyl bromide.
(ii) (±) 2-Butanol is optically inactive.
(iii) C—X bond length in halobenzene is smaller than C—X bond length in CH3 —X.
Answer:
(i) Since I– ion is a better leaving group than Br– ion, hence, CH3I reacts faster than CH3Br in SN2 reaction with OH– ion.
(ii) (±) 2-Butanol is a racemic mixture, i.e., there are two enantiomers in equal proportions. The rotation by one enantiomer will be cancelled by the rotation due to the other isomer, making the mixture optically inactive.
(iii) In CH3—X the carbon atom is sp2-hybridised while in halobenzene the carbon atom is sp3-hybridised. The sp2-hybridised carbon is more electronegative due to greater s-character and holds the electron pair of C—X bond more tightly than sp3-hybridised carbon with less s-character. Thus, C—X bond length in CH3—X is bigger than C—X in halobenzene.
Question 2.
Give reasons for the following:
(i) Haloalkanes easily dissolve in organic solvents.
(ii) Halogen compounds used in industry as solvents are alkyl chlorides rather than bromides and iodides.
Answer:
(i) Haloalkanes dissolve in organic solvents because the new intermolecular attractions between haloalkanes and organic solvent molecules have much the same strength as ones being broken in the separate haloalkanes and solvent molecules.
(ii) Because alkyl chlorides are more stable and more volatile than bromides and iodides.
![]()
Question 3.
Write the mechanism of the following reaction
![]()
Answer:
SN2 mechanism
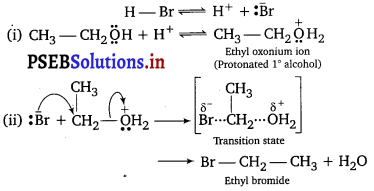
Question 4.
Which would undergo SN1 reaction faster in the following pairs and why ?
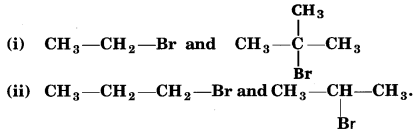
Answer:
(i) 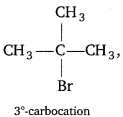
Tertiary halide reacts faster than primary halide because of greater stability of 3°-carbocation.
(ii) 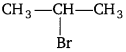
Because the secondary carbocation formed in the slowest step is more stable than the primary carbocation.
Question 5.
Give reasons:
(i) n-Butyl bromide has higher boiling point than f-butyl bromide. Racemic mixture is optically inactive.
(ii) The presence of nitro group (—NO2) at o/p positions increases the reactivity of haloarenes towards nucleophilic substitution reactions.
Answer:
(i) n-butyl bromide being a straight chain alkyl halide has larger surface area than tert butyl bromide. Larger the surface area, larger the magnitude of the van der Waal’s forces and hence higher is the boiling point.
(ii) A racemic mixture contains the two enantiomers d and l in equal •proportions. As the rotation due to one enantiomer is cancelled by equal and opposite rotation of another enantiomer, therefore, it is optically inactive.
(iii) The presence of NO2 group at o/p position in haloarenes helps in the stabilisation of resulting carbanion by -R and -I effects and hence increases the reactivity of haloarenes towards nucleophilic substitution reactions.
![]()
Question 6.
(i) Why are alkyl halides insoluble in water ?
(ii) Why is butan-l-ol optically inactive but butan-2-ol is optically active ?
(iii) Although chlorine is an electron withdrawing group, yet it is ortho, para directing in electrophilic aromatic substitution reactions. Why ?
Answer:
(i) This is due to the inability of alkyl halide molecule to form intermolecular hydrogen bonds with water molecules.
(ii) 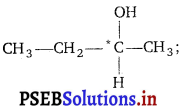
due to presence of a chiral carbon butan-2-ol is an optically active compound.
(iii) As the weaker resonance (+R) effect of Cl which stabilise the carbocation formed tends to oppose the stronger inductive (-I) effect of Cl which destabilise the carbocation at ortho and para positions and makes deactivation less for ortho and para position.
Question 7.
(i) Allyl chloride can be distinguished from vinyl chloride by NaOH and silver nitrate test. Comment.
(ii) Alkyl halide reacts with lithium aluminium hydride to give alkane. Name the attacking reagent which will bring out this change.
Answer:
(i) Vinyl chloride does not respond to NaOH and silver nitrate test because of partial double bond character due to resonance.
(ii) Hydride ion (H )
Question 8.
Give the ITJPAC name of the product formed when :
(i) 2-Methyl-l-bromopropane is treated with sodium in the presence of dry ether.
(ii) 1-Methyl cyclohexene is treated with HI.
(iii) Chloroethane is treated with silver nitrite.
Answer:
(i) 2, 5-dimethylhexane
(ii) 1 -Methyl-1 -iodocyclohexane
(iii) Nitroethane
![]()
Long Answer Type Questions
Question 1.
Some alkyl halides undergo substitution whereas some undergo elimination reaction on treatment with base. Discuss the structural features of alkyl halides with the help of examples which are responsible for this difference.
Answer:
Primary alkyl halides follow SN2 mechanism in which a nucleophile attacks at 180° to the halogen atom. A transition state is formed in which carbon is bonded to two nucleophiles and finally halogen atom is pushed out. In SN2 mechanism, substitution of nucleophile takes place as follows :
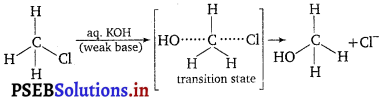
Thus, in SN2 mechanism, substitution takes place. Tertiary alkyl halides follow? SN1 mechanism, In this case, tert-alkyl halides form 3° carbocation. Now, if the reagent used is a weak base then substitution occur while if it is a strong base then instead of substitution elimination occur.
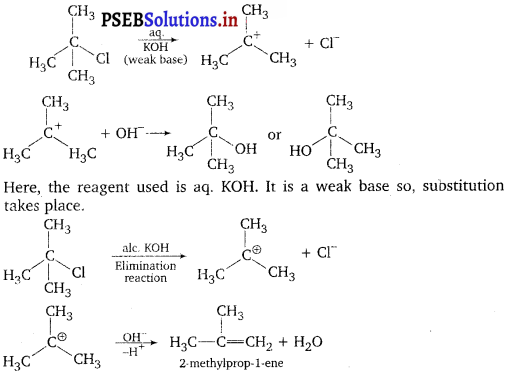
As alc. KOH is a strong base, so elimination competes over substitution and alkene is formed.
![]()
Question 2.
Why are aryl halides less reactive towards nucleophilic substitution reactions than alkyl halides ? How can we enhance the reactivity of aryl halides ?
Answer:
Aryl halides are less reactive towards nucleophilic substitution reaction due to the following reasons :
(i) In haloarenes, the lone pair of electron on halogen are in resonance with benzene ring. So, C—Cl bond acquires partial double bond character which strengthen C—Cl bond. Therefore, they are less reactive towards nucleophilic substitution reaction.
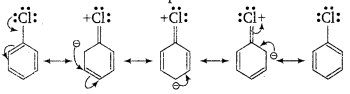
(ii) In haloarenes, the carbon atom attached to halogen is sp2-hybridised. The sp2-hybridised carbon is more electronegative than sp3-hybridised carbon. This sp 2-hybridised carbon in haloarenes can hold the electron pair of C—X bond more tightly and make this C—Cl bond shorter than C—Cl bond haloalkanes.
(iii) Since, it is difficult to break a shorter bond than a longer bond therefore haloarenes are less reactive than haloarenes.
In haloarenes, the phenyl cation will not be stabilised by resonance therefore SN1 mechanism ruled out.
(iv) Because of the repulsion between the nucleophile and electron rich arenes, aryl halides are less reactive than alkyl halides.
The reactivity of aryl halides can be increased by the presence of an electron withdrawing group (—NO2) at ortho and para positions. However, no effect on reactivity of haloarenes is observed by the presence of electron withdrawing group at meta-position. Mechanism of the reaction is as depicted with OH– ion.
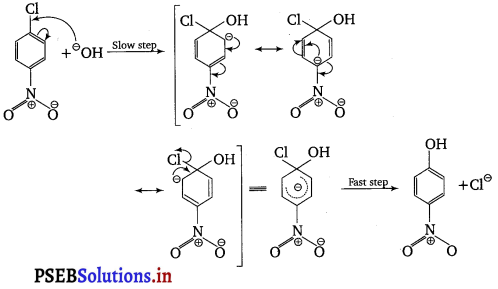
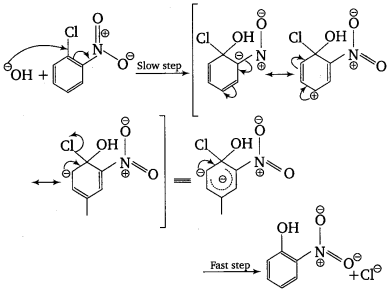
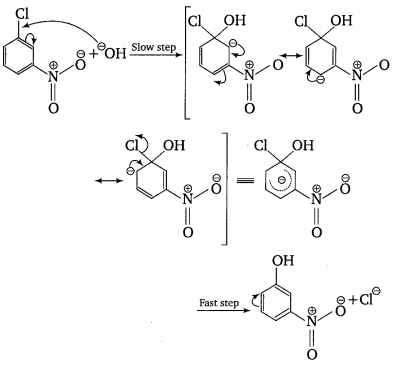
From the above resonance, it is very clear that electron density is rich at ortho and para positions. So, presence of EWG will facilitate nucleophilic at ortho and para positions not on meta position.
