Punjab State Board PSEB 12th Class Chemistry Important Questions Chapter 11 Alcohols, Phenols and Ethers Important Questions and Answers.
PSEB 12th Class Chemistry Important Questions Chapter 11 Alcohols, Phenols and Ethers
Very Short Answer Type Questions
Question 1.
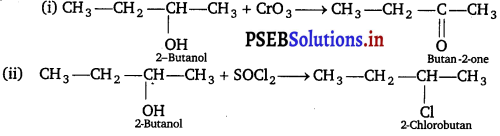
Write the structures of the products when Butan-2-ol reacts with the following:
(i) CrO3
(ii) SOCl2
Answer:
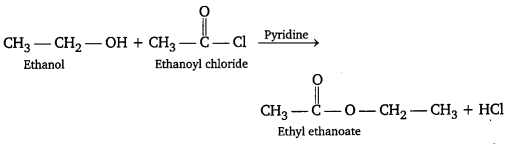
Question 2.
What happens when ethanol reacts with CH3COCl/pyridine ?
Answer:
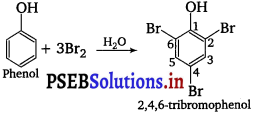
Question 3.
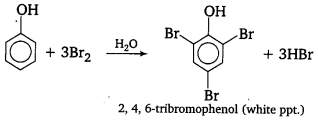
When phenol is created with bromine water, while precipitate is obtained. Prove the structure and the name of the compound formed.
Answer:
When phenol is treated with bromine water, white ppt. of 2, 4, 6-tribromophenol is obtained.
![]()
Question 4.
Answer the following questions :
(i) Dipole moment of phenol is smaller than that of methanol. Why?
(ii) In Kolbe’s reaction, instead of phenol, phenoxide ion is treated with carbon dioxide. Why ?
Answer:
(i) In phenol, C—O bond is less polar due to electron-withdrawing effect of benzene ring whereas in methanol, C—O bond is more polar due to electron-releasing effect of —CH3 group.
(ii) Phenoxide ion is more reactive than phenol towards electrophilic aromatic substitution and hence undergoes electrophilic substitution with carbon dioxide which is a weak electrophile.
Question 5.
What is denatured alcohol ?
Answer:
Alcohol is made unfit for drinking by mixing some copper sulphate and pyridine in it. This is called denatured alcohol.
Question 6.
Arrange the following compounds in the increasing order of their acidic strength: p-cresol, p -nitrophenol, phenol
Answer:
p-cresol < phenol < p-nitrophenol
![]()
Question 7.
Arrange the following compounds in decreasing order of acidity.
(i) H2O, ROH, HC ☰ CH
(ii) ![]()
(iii) CH3OH, H2O, C6H6OH
Answer:
(i) H2O > ROH > HC ☰ CH
(ii) ![]()
(iii) C6H5OH > H2O > CH3OH
Question 8.
Suggest a reagent for conversion of ethanol to ethanal.
Answer:
Ethanol can be oxidises into ethanal by using pyridinium chlorochromate.
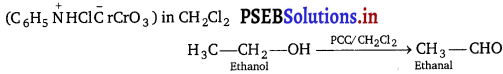
Question 9.
Explain why sodium metal can be used for drying diethyl ether but not ethyl alcohol.
Answer:
Due to presence of an active hydrogen atom, ethyl alcohol reacts with sodium metal.
2CH3 — CH2 — OH + 2Na → 2CH3 — CH2 — ONa + H2
Diethyl ether, on the other hand, does not have replaceable hydrogen atom therefore does not react with sodium metal hence can be dried by metallic sodium.
![]()
Question 10.
Phenol is an acid but does not react with sodium bicarbonate solution. Why?
Answer:
Phenol is a weaker acid than carbonic acid (H2CO3) and hence does not liberate CO2from sodium bicarbonate.
Question 11.
In the process of wine making, ripened grapes are crushed so that sugar and enzyme should come in contact with each other and fermentation should start. What will happen if anaerobic conditions are not maintained during this process?
Answer:
Ethanol will be converted into ethanoic acid.
Short Answer Type Questions
Question 1.
Why is the reactivity of all the three classes of alcohols with cone. HCl and ZnCl2 (Lucas reagent) different ?
Answer:
The reaction of alcohols with Lucas reagent (cone. HCl and ZnCl2) follow SN1 mechanism. SN1 mechanism depends upon the stability of carbocations (intermediate). More stable the intermediate carbocation, more reactive is the alcohol.
Tertiary carbocations are most stable among the three classes of carbocations and the order of the stability of carbocation is 3° > 2° > 1°. This order, intum, reflects the order of reactivity of three classes of alcohols i. e., 3° > 2° > 1°.
Thus , as the stability of carbocations are different so the reactivity of all the three classes of alcohols with Lucas reagent is different.
![]()
Question 2.
Write the mechanism of the following reaction:
![]()
Answer:
SN2 mechanism

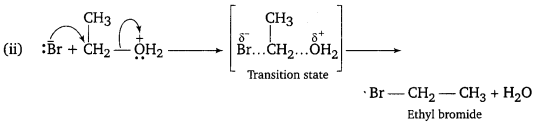
Question 3.
Explain a process in which a biocatalyst is used in industrial preparation of a compound known to you.
Answer:
Enzymes are biocatalyst. These biocatalysts (enzymes) are used in the industrial preparation of ethanol. Ethanol is prepared by the fermentation of molasses—a dark brown coloured syrup left after crystallisation of sugar which still contains about 40% of sugar.
The process of fermentation actually involves breaking down of large molecules into simple ones in the presence of enzymes. The source of these enzymes is yeast. The various reactions taking place during fermentation of carbohydrates are :
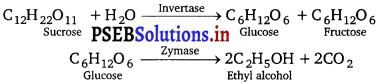
In wine making, grapes are the source of sugars and yeast. As grapes ripen, the quantity of sugar increases and yeast grows on the outer skin. When grapes are crushed, sugar and the enzyme come in contact and fermentation starts. Fermentation takes place in anaerobic conditions i.e., in absence of air. CO2 gas is released during fermentation.
The action of zymase is inhibited once the percentage of alcohol ,formed exceeds 14 per cent. If air gets into fermentation mixture, the oxygen of air oxidises ethanol to ethanoic acid which in turn destroys the taste of alcoholic drinks.
![]()
Question 4.
Explain why alcohols and ethers of comparable molecular mass have different boiling points ?
Answer:
Boiling point depends upon the strength of intermolecular forces of attraction. Higher these forces of attraction, more will be the boiling point. Alcohols undergo intermolecular hydrogen bonding. So, the molecules of alcohols are held together by strong intermolecular forces of attraction.
But in ethers no hydrogen atom is bonded to oxygen. Therefore, ethers are held together by weak dipole-dipole forces, not by strong hydrogen bond.
Since, lesser amount of energy is required than to break weak dipole-dipole forces in ethers than to break strong hydrogen bonds in alcohol.
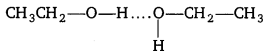
Question 5.
Explain why is O = C = O non-polar while R—O—R is polar ?
Answer:
CO2 is a linear molecule. The dipole moment of two C —O bonds are equal and opposite and they cancel each other and hence the dipole moment of CO2 is zero and it is a non-polar molecule.
![]()
While for ethers, two dipoles are pointing in the same direction. These two dipoles do not cancel the effect of each other. Therefore, there is a finite resultant dipoles and hence R—O—R is a polar molecule.
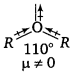
Question 6.
Give reasons for the following:
(i) p-Nitrophenol is more acidic than o-nitrophenol
(ii) Bond angle C—O—C in ethers is slightly higher than the tetrahedral angle (109°28′).
(iii) (CH3)3C—Br on reaction with NaOCH3 gives an alkene instead of an ether.
Answer:
(i) 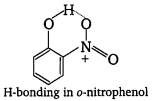
Intramolecular H-bonding in o-nitrophenol makes loss of proton difficult. Therefore, p-nitrophenol is more acidic than o-nitrophenol.
(ii) The ![]() bond angle in ether is slightly higher than 109 °28′ due to repulsive interaction between the two bulky alkyl groups.
bond angle in ether is slightly higher than 109 °28′ due to repulsive interaction between the two bulky alkyl groups.
(iii) It is because NaOCH3 is a strong nucleophile as well as a strong base. Thus, elimination reaction predominates over substitution reaction.
![]()
Question 7.
Explain the following behaviours :
(i) Alcohols are more soluble in water than the hydrocarbons of comparable molecular masses.
(ii) Ortho-nitrophenol is more acidic than ortho-methoxyphenol.
(iii) Cumene is a better starting material for the preparation of phenol.
Answer:
(i) Alcohols are more soluble in water than the hydrocarbons of comparable molecular masses because of H-bond formation between alcohol and water molecules.
(ii) Ortho-nitrophenol is more acidic than ortho-methoxyphenol because nitro being the electron with drawing group stabilises the phenoxids ion.
(iii) Cumene is a better starting material for the preparation of phenol because side product formed in this reaction is acetone which is another important organic compound.
Long Answer Type Questions
Question 1.
(a) Name the starting material used in the industrial preparation of phenol.
(b) Write complete reaction for the bromination of phenol in aqueous and non-aqueous medium.
(c) Explain why Lewis acid is not required in bromination of phenol?
Answer:
(a) The starting material used in the industrial preparation of phenol is cumene.
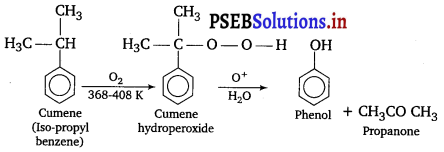
(b) Phenols when treated with bromine water gives polyhalogen derivatives in which all the hydrogen atoms present at ortho and para positions with respect to —OH group are replaced by bromine atoms.
However, in non-aqueous medium such as CS2, CCl4, CHCl3 monobromophenols are obtained.
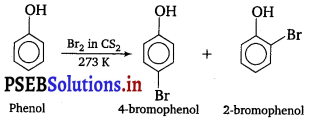
In aqueous solution, phenol ionises to form phenoxide ion. This ion activates the benzene ring to a very large extent and hence the substitution of halogen takes place at all three positions.
On the other hand, in non-aqueous solution ionisation of phenol is greatly suppressed. Therefore, ring is activated slightly and hence monosubstitution occur.
(c) Lewis acid is an electron deficient molecule. In bromination of benzene, Lewis acid is used-to polarise Br2 to form Br+ electrophile.
In case of phenol, oxygen atom of phenol itself polarises the bromine molecule to form Br+ ion (electrophile). So, Lewis acid is not required in the bromination of phenol.
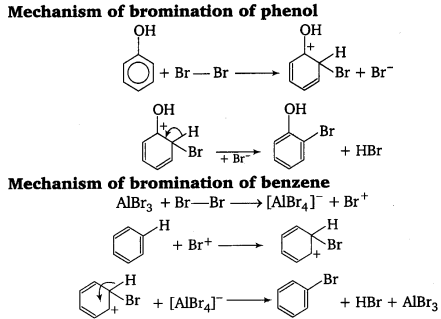
![]()
Question 2.
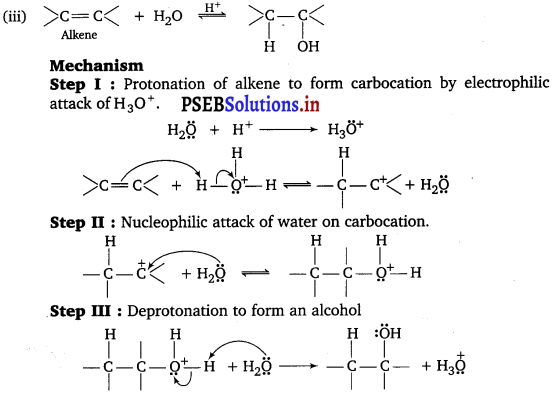
Explain the mechanism of the following reactions :
(i) Addition of Grignard’s reagent to the carbonyl group of a compound forming an adduct followed by hydrolysis.
(ii) Acid catalysed dehydration of an alcohol forming an alkene.
(iii) Acid catalysed hydration of an alkene forming an alcohol.
Answer:
(i) Step I : Nucleophilic addition of Grignard reagent to carbonyl group.
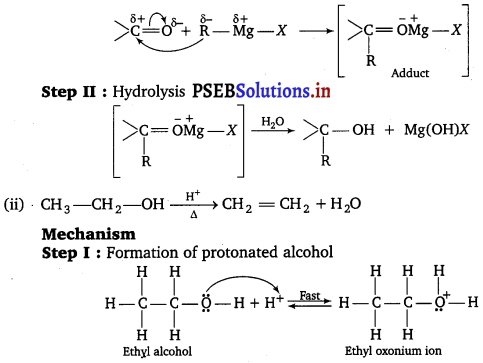
Step II : Formation of carbocation : It is the slowest step and hence, the rate determining step.
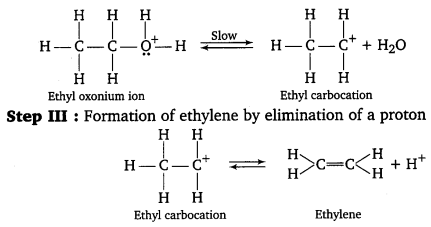
To drive the equilibrium to the right, ethylene is removed as it is formed.