Punjab State Board PSEB 12th Class Chemistry Important Questions Chapter 4 Chemical Kinetics Important Questions and Answers.
PSEB 12th Class Chemistry Important Questions Chapter 4 Chemical Kinetics
Very Short Answer Type Questions
Question 1.
For which type of reactions, order and molecularity have the same value?
Answer:
If the reaction is an elementary reaction, order is same as molecularity.
Question 2.
Why is the probability of reaction with molecularity higher than three very rare?
Answer:
The probability of more than three molecules colliding simultaneously is very small. Hence, possibility of molecularity being three is very low.
Question 3.
State a condition under which a bimolecular reaction is kinetically first order.
Answer:
A bimolecular reaction may become kinetically of first order if one of the reactants is in excess.
![]()
Question 4.
Thermodynamic feasibility of the reaction alone cannot decide the rate of the reaction. Explain with the help of one example.
Answer:
Thermodynamically the conversion of diamond to graphite is highly feasible but this reaction is very slow because its activation energy is high.
Question 5.
Why is it that instantaneous rate of reaction does not change when a part of the reacting solution is taken out?
Answer:
Instantaneous rate is measured over a very small interval of time, hence, it does not change when a part of solution is taken out.
Question 6.
A reaction is 50% complete in 2 hours and 75% complete in 4 hours. What is the order of the reaction?
Solution:
As t75% = 2t50%
Therefore, it is a first order reaction.
![]()
Question 7.
Define threshold energy of a reaction.
Answer:
Threshold energy is the minimum energy which must be possessed by reacting molecules in order to undergo effective collision which leads to formation of product molecules.
Question 8.
Why does the rate of a reaction increase with rise in temperature?
Answer:
At higher temperatures, larger fraction of colliding particles can cross the energy barrier (i.e., the activation energy), which leads to faster rate.
Question 9.
What is the difference between rate law and law of mass action?
Answer:
Rate law is an experimental law. On the other hand, law of mass action is a theoretical law based on the balanced chemical reaction.
![]()
Question 10.
What do you understand by ‘Rate of reaction’?
Answer:
The change in the concentration of any one of the reactants or products per unit time is termed as the rate of reaction.
Question 11.
In the Arrhenius equation, what does the factor e a corresponds to?
Answer:
e-Ea/RT corresponds to the fraction of molecules that have kinetic energy greater than Ea,
Short Answer Type Questions
Question 1
What do you understand by the ‘order of a reaction’? Identify the reaction order from each of the following units of reaction rate constant:
(i) L-1mols-1
(ii) Lmol-1s-1.
Solution:
The sum of powers of the concentration of the reactants in the rate law expression is called order of reaction.
For a general reaction: aA + bB → Products
If rate = k[A]m [B]n; order of reaction = m + n
(i) General unit of rate constant, k = (mol L-1 )1-ns-1
L-1mol s-1 = (mol L-1 )1-ns-1
-1 = -1 + n ⇒ n = 0 ∴ Reaction order = 0
(ii) L mol-1 s-1 = (mol L-1)1-n s-1
1 = -1 + n ⇒ n = 2 ∴ Reaction order = 2
![]()
Question 2.
The rate constant for the first order decomposition of H2O2 is given by the following equation :
log k = 14.2 – \(\frac{1.0 \times 10^{4}}{T}\)K
Calculate Ea for this reaction and rate constant k if its half-life period be 200 minutes.
(Given: R = 8.314 JK-1 mol-1)
Solution:
Comparing the equation, log k = 14.2 – \(\frac{1.0 \times 10^{4}}{T}\) K with the equation,
log k = log A = \(\frac{E_{a}}{2.303 R T}\), we get
\(\frac{E_{a}}{2.303 R}\) = 1.0 × 104 K or Ea = 1.0 × 104 K × 2.303 × R
Ea = 1.0 × 104 K × 2.303 × 8.314 JK-1
= 19.1471 × 104 Jmol-1
= 191.47 kJ mol-1
For a first order reaction, tt/2 = \(\frac{0.693}{k}\) or k = \(\frac{0.693}{t_{1 / 2}}\)
k = \(\frac{0.693}{200 \mathrm{~min}}\) = 3.465 × 10 -3min-1
Question 3.
The reaction, N2(g) + O2(g) ⇌ 2NO(g) contributes to air pollution whenever a fuel is burnt in air at a high temperature. At 1500 K, equilibrium constant K for it is 1.0 × 10-5. Suppose in a case [N2] = 0.80 mol L-1 and [O2] = 0.20 mol L-1 before any reaction occurs. Calculate the equilibrium concentrations of the reactants and the product after the mixture has been heated to 1500 K.
Solution:
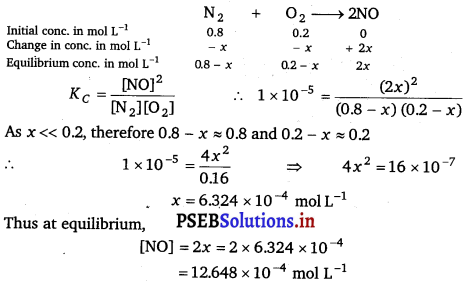
[N2] = 0.8 – 6.324 × 104 mol L-1
= 0.799 molL-1
[O2] = 0.2 – 6.324 × 10-4 mol L-1
= 0.199 mol L-1
![]()
Question 4.
For a general reaction, A → B, plot of concentration of A vs time is given in figure. Answer the following questions on the basis of this graph.
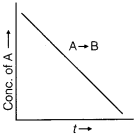
(i) What is the order of the reaction?
(ii) What is the slope of the curve?
(iii) What are the units of rate constant?
Answer:
(i) Zero order
(ii) Slope = – k
(iii) Units of rate constant = mol L-1 s-1
Question 5.
For a reaction, A + B → products, the rate law is rate = k [A][B]a3/2. Can the reaction be an elementary reaction? Explain.
Answer:
During an elementary reaction, the number of atoms or ions colliding to react is referred to as molecularity. Had this been an elementary reaction the order of reaction with respect to B would have been 1, but in the given rate law it is \(\frac{3}{2}\). This indicated that the reaction is not an elementary reaction.
![]()
Question 6.
The rate constant for a first order reaction is 60 s-1. How much time will it take to reduce 1 g of the reactant to 0.0625 g?
Answer:
We know that, t = \(\frac{2.303}{k}\) log \(\frac{[R]_{0}}{[R]}\)
t = \(\frac{2.303}{60}\) log \(\frac{1}{0.0625}\)
t = 0.0462 s
Long Answer Type Questions
Question 1.
For the hydrolysis of methyl acetate in aqueous solution, the following results were obtained:
| t/s | 0 | 30 | 60 |
| [CH3COOCH3]/mol L-1 | 0.60 | 0.30 | 0.15 |
(i) Show that it follows pseudo first order reaction, as the concentration of water remains constant.
(ii) Calculate the average rate of reaction between the time interval 30 to 60 seconds.
(Given log 2 = 0.3010, log 4 = 0.6021)
Solution:
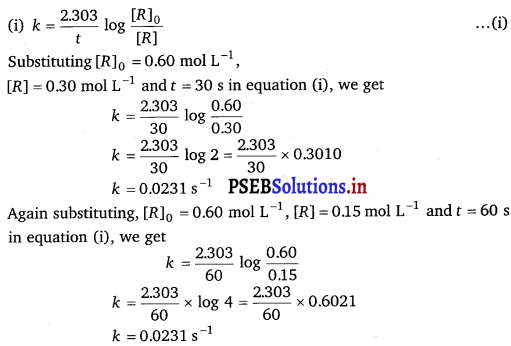
As the value of k is same in both the cases, therefore, hydrolysis of methylacetate in aqueous solution follows pseudo first order reaction.
(ii) Average rate = \(-\frac{\Delta\left[\mathrm{CH}_{3} \mathrm{COOCH}_{3}\right]}{\Delta t}\)
= \(\frac{-[0.15-0.30]}{60-30}\) = \(\frac{0.15}{30}\)
Average rate = 0.005 mol L-1s-1
![]()
Question 2.
Describe how does the enthalpy of reaction remain unchanged when a catalyst is used in the reaction?
Answer:
A catalyst is a substance which increases the speed of a reaction without itself undergoing any chemical change.
According to “intermediate complex formation theory” reactants first combine with the catalyst to form an intermediate complex which is short-lived and decomposes to form the products and regenerating the catalyst.
The intermediate formed has much lower potential energy than the intermediate complex formed between the reactants in the absence of the catalyst.
Thus, the presence of catalyst lowers the potential energy barrier and the reaction follows a new alternate pathway which require less activation energy.
We know that, lower the activation energy, faster is the reaction because more reactant molecules can cross the energy barrier and change into products.
Enthalpy, △H is a ‘state function. Enthalpy of reaction, i.e., difference in energy between reactants and product is constant, which is clear from potential energy diagram.
Potential energy diagram of catalysed reaction is given as:
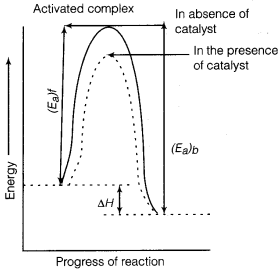
![]()
Question 3.
All energetically effective collisions do not result in a chemical change. Explain with the help of an example.
Answer:
Only effective collision lead to the formation of products. It means that collisions in which molecules collide with sufficient kinetic energy (called threshold energy = activation energy + energy possessed by reacting species).
And proper orientation lead to a chemical change because it facilitates the breaking of old bonds between (reactant) molecules and formation of the new ones i.e., in products.
e.g., formation of methanol from bromomethane depends upon the orientation of the reactant molecules.
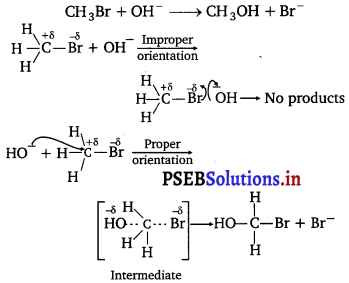
The proper orientation of reactant molecules leads to bond formation whereas improper orientation makes them simply back and no products are formed.
To account for effective collisions, another factor P (probability of steric factor) is introduced K = PZABe-Ea/RT.
