Punjab State Board PSEB 12th Class Physics Book Solutions Chapter 11 Dual Nature of Radiation and Matter Textbook Exercise Questions and Answers.
PSEB Solutions for Class 12 Physics Chapter 11 Dual Nature of Radiation and Matter
PSEB 12th Class Physics Guide Dual Nature of Radiation and Matter Textbook Questions and Answers
Question 1.
Find the
(a) maximum frequency, and
(b) minimum wavelength of X-rays produced by 30 kV electrons.
Answer:
Potentialoftheelectrons, V=30 kV= 3O x 103 V=3 x 104 V
Hence, energy of the electrons, E = 3 x 104 eV
where, e = Charge on an electron = 1.6 x 10-19C
(a) Maximum frequency produced by the X-rays = v
The energy of the electrons is given by the relation
E=eV=hv
where, h = Planck’s constant = 6.63 x 10-34 Js
∴ v = \(\frac{e V}{h} \) (∵ E = eV)
= \(\frac{1.6 \times 10^{-19} \times 3 \times 10^{4}}{6.63 \times 10^{-34}}\) = 7.24 x 1018 Hz
Hence, the maximum frequency of X-rays produced is 7.24 x 1018 Hz
(b) The minimum wavelength produced by the X-rays is given as
λ = \(\frac{c}{v}\)
= \(\frac{3 \times 10^{8}}{7.24 \times 10^{18}}\)
= 0.414 x 10-10
= 0.0414 x 10-9 m
= 0.0414 nm
Hence, the minimum wavelength of X-rays produced is 0.0414 nm.
Question 2.
The work function of caesium metal is 2.14 eV. When light of frequency 6 x 1014 Hz is incident on the metal surface, photoemission of electrons occurs.
What is the
(a) maximum kinetic energy of the emitted electrons,
(b) stopping potential, and
(c) maximum speed of the emitted photoelectrons?
Answer:
Work function of caesium metal, Φ0 = 2.14 eV
Frequency of light, v = 6.0 x 1014 Hz
The maximum kinetic energy is given by the photoelectric effect as
K = hv- Φ0
where, h = Planck’s constant = 6.63 x 10-34 Js .
∴ k = \(\frac{6.63 \times 10^{-34} \times 6 \times 10^{14}}{1.6 \times 10^{-19}}-2.14 \)
( ∵ e=1.6 x 10-19)
= 2.485-2.140 =0.345eV
Hence, the maximum kinetic energy of the emitted electrons is 0.345 eV.
(b) For stopping potential V0, we can write the equation for kinetic energy
as K=eV0
∴ V0 = \(\frac{K}{e}\) (∵ e=1.6×1019)
= \(\frac{0.345 \times 1.6 \times 10^{-19}}{1.6 \times 10^{-19}}\) =0.345V
Hence, the stopping potential of the material is 0.345 V.
(c) Maximum speed of the emitted photoelectrons = y
Hence, the relation for kinetic energy can be written as
K = \(\frac{1}{2}\) mv2
where, m = mass of an electron = 9.1 x 10-31 kg
(∴ e=1.6 x 10-19)
= \(\frac{2 \times 0.345 \times 1.6 \times 10^{-19}}{9.1 \times 10^{-31}}\) = 0.1104 x 1012
∴ v = 3.323 x 105 m/s = 332.3 km/s
Hence, the maximum speed of the emitted photoelectrons is 332.3 km/s.
![]()
Question 3.
The photoelectric cut off voltage n a certain experiment is 1.5 V. What is the maximum kinetic energy of photoelectrons emitted?
Answer:
Cut-off voltage, V0 = 1.5 V
Maximum kinetic energy of photoelectrons
EK =eV0 =1.5eV=1.5 x 1.6 x 10-19J
=2.4 x 10-19J.
Question 4.
Monochromatic light of wavelength 632.8 mn is produced by a helium-neon laser. The power emitted is 9.42 mW.
(a) Find the energy and momentum of each photon in the light beam,
(b) How many photons per second, on the average, arrive at a target irradiated by this beam? (Assume the beam to have uniform cross-section which is less than the target area), and
(c) How fast does a hydrogen atom have to travel in order to have the same momentum as that of the photon?
Answer:
Wavelength of the monochromatic light, 632.8 nm = 632.8 x 10-9 m
Power emitted by the laser, P = 9.42 mW = 9.42 x 10-3 W
Planck’s constant, h = 6.63 x 10-34Js
Speed of light, c=3 x 108 m/s
Mass of a hydrogen atom, m =1.66 x 10-27 kg
(a) The energy of each photon is given as
E = \(\frac{h c}{\lambda}\)
= \(\frac{6.63 \times 10^{-34} \times 3 \times 10^{8}}{632.8 \times 10^{-9}}\)
= 3.141 x 10-19
The momentum of each photon is given as
p = \(\frac{h}{\lambda}\)
= \(\frac{6.63 \times 10^{-34}}{632.8 \times 10^{-9}}=1.047 \times 10^{-27} \mathrm{~kg} \mathrm{~ms}^{-1} \)
(b) Number of photons arriving per second, at a target irradiated by the beam = n.
Assume that the beam has a uniform cross-section that is less than the
target area.
Hence, the equation for power can be written as
P=nE
∴ n= \(\frac{P}{E}\)
= \(\frac{9.42 \times 10^{-3}}{3.141 \times 10^{-19}}\) = 3 x 1016
(c) Momentum of the hydrogen atom is the same as the momentum of the photon, .
p=1.047 x 1027 kgms-1
Momentum is given as
p = mv
where, v = speed of the hydrogen atom
v = \(\frac{p}{m}\)
= \(=\frac{1.047 \times 10^{-27}}{1.66 \times 10^{-27}}\) = 0.630m/s
Question 5.
The enery flux of sunlight reaching the surface of the earth is 1.388 x 103 W/m2.
How many photons (nearly) per square metre are incident on the Earth per second? Assume that the photons in the sunlight have an average wavelength of 550 rims.
Answer:
Energy flux of sunlight reaching the surface of earth,
Φ = 1.388 x 103 W/m2
Hence, power of sunlight per square metre, P = 1.388 x 103W
Speed of light, c = 3 x 108 m/s
Planck’s constant, h = 6.63 x 10-34 Js
Average wavelength of photons present in sunlight, λ = 550 nm.
=550 x 10-9m
Number of photons per square metre incident on earth per second = n
Hence, the equation for power can be written as
P = nE
∴ n = \(\frac{P}{E}=\frac{P \lambda}{h c}=\frac{1.388 \times 10^{3} \times 550 \times 10^{-9}}{6.63 \times 10^{-34} \times 3 \times 10^{8}}\)
= 3.84 x 1021 photons/m2/s
Therefore, every second, 3.84×1021 photons are incident per square metre on earth.
![]()
Question 6.
In an experiment on photoelectric effect, the slope of the cut-off voltage versus frequency of incident light is found to be 4.12 x 10-15 Vs. Calculate the value of Planck’s constant.
Answer:
The slope of the cut-off voltage (V) versus frequency (v) of an incident light is given as
\(\frac{V}{v}\) = 4.12 x 10-15 Vs
V is related to frequency by the equation
hv = eV
where, e = charge on an electron = 1.6 x 10-19
h = Planck’s constant
∴ h = e x \(\frac{V}{v}\)
= 1.6 x 10-19 x 4.12 x 10-15
= 6.592 x 10-34 Js
Therefore, the value of Planck’s constant is 6.592 x 10-34 Js.
Question 7.
A 100 W sodium lamp radiates energy uniformly in all directions. The lamp is located at the centre of a large sphere that absorbs all the sodium light which is incident on it. The wavelength of the sodium light is 589 nm.
(a) What is the energy per photon associated with the sodium light?
(b) At what rate are the photons delivered to the sphere?
Answer:
Power of the sodium lamp,. P = 100 W
Wavelength of the emitted sodium light, λ = 589 nm = 589 x 109 m
Planck’s constant, h = 6.63 x 10-34 Js
Speed of light, c = 3 x 108 m/s
The energy per photon associated with the sodium light is given as
E= \(\frac{h c}{\lambda}\)
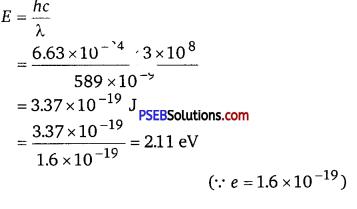
(b) Number of photons delivered to the sphere = n
The equation for power can be written as
P=nE
∴ n = \(\frac{P}{E}=\frac{100}{3.37 \times 10^{-19}}\) = 2.96 x 1020 photons/s
Therefore, every second, 2.96 x 1020 photons are delivered to the sphere.
Question 8.
The threshold frequency for a certain metal is 3.3 x 1014 Hz. If light of frequency 8.2 x 1014 Hz is incident on the metal, predict the cut-off voltage for the photoelectric emission.
Answer:
Threshold frequency of the metal, v0 = 3.3 x 1014 Hz
Frequency of light incident on the metal, v = 82 x 1014 Hz
Charge on’an electron, e = 1.6 x 10-19 C .
Planck’s constant, h = 6.63 x 10-34 Js
Cut-off voltage for the photoelectric emission from the metal = V0
The equation for the cut-off energy is given as
eV0 = h(v-v0)
Vo = \(\frac{h\left(v-v_{0}\right)}{e}\)
= \(\frac{6.63 \times 10^{-14} \times\left(8.2 \times 10^{14}-3.3 \times 10^{14}\right)}{1.6 \times 10^{-19}}\)
= 2.0292 V
Therefore, the cut-off voltage for the photoelectric emission is 2.0292 V.
Question 9.
The work function for a certain metal Is 4.2 eV. Will this metal give photoelectric emission for incident radiation of
wavelength 330 nm?
Answer:
The energy of incident radiations
E = \(\frac{h c}{\lambda}\) = \(\frac{6.63 \times 10^{-34} \times 3 \times 10^{8}}{330 \times 10^{-9}}\) J
= 6.03 x 10-19 J
= \(\frac{6.03 \times 10^{-19}}{1.6 \times 10^{-19}}\)eV = 3.77 eV
The work function of photometal, Φ0 = 4.2 eV
As energy of incident photon is less than work function, photoemission is not possible.
![]()
Question 10.
Light of frequency 7.21 x 1014 Hz is incident on a metal surface. Electrons with a maximum speed of 6.0 x10s m/s are ejected from the surface. What is the threshold frequency for photoemission of electrons?
Answer:
Frequency of light incident on the metal surface, v = 7.21 x 1014 Hz
Maximum speed of the electrons, v = 6.0 x 105 m/s
Planck’s constant, h = 6.63 x 10-34 Js
Mass of an electron, m = 9.1 x 10-31 kg
For threshold frequency v0, the relation for kinetic energy is written asFor threshold frequency y0, the relation for kinetic energy is written as
\(\frac{1}{2} m v^{2}\) = h(v-v0)
v0 = v – \(\frac{m v^{2}}{2 h}\)

Therefore, the threshold frequency for the photoemission of electrons is 4.738 x 1014 Hz.
Question 11.
Light of wavelength 488 mn is produced by an argon laser which is used in the photoelectric effect. When light from this spectral line is incident on the emitter, the stopping (cut-off) potential of photoelectrons is 0.38 V. Find the work function of the material from which the emitter is made.
Answer:
Wavelength of light produced by the argon laser,
λ = 488 nm = 488 x 10-9 m
Stopping potential of the photoelectrons, V0 = 0.38 V
1 eV=l.6 x 10-19 J
∴ V0= \(\frac{0.38}{1.6 \times 10^{-19}}\) eV
Planck’s constant, h = 6.63 x 10-34 Js
Charge on an electron, e = 1.6 x 10-19 C
Speed of light, c =3 x 10 m/s
From Einstein’s photoelectric effect, we have the relation involving the work function Φ0 of the material of the emitter as

Therefore, the material with which the emitter is made has the work function of 2.16 eV.
Question 12.
Calculate the
(a) momentum, and
(b) de Broglie wavelength of the electrons accelerated through a potential difference of 56 V.
Answer:
Potential difference, V = 56 V
Planck’s constant, h = 6.63 x 10-34 Js
Mass of an electron, m = 9.1 x 10-31 kg
Charge on an electron, e = 1.6 x 10-19 C
(a) At equilibrium, the kinetic energy of each electron is equal to the accelerating potential, i.e., we can write the relation for velocity (v) of each electron as
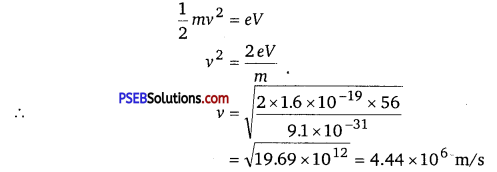
The momentum of each accelerated electron is given as
P = mv
= 9.1 x 10-31 x 4.44 x 106
= 4.04 x 10-24 kg m s-1
Therefore, the momentum of each electron is 4.04 x 10-24 kg m s-1.
(b) de Broglie wavelength of an electron accelerating through a potential V is given by the relation

Therefore, the de Broglie wavelength of each electron is 0.1639 nm.
![]()
Question 13.
What is the
(a) momentum,
(b) speed, and
(c) de Broglie wavelength of an electron with kinetic energy of 120 eV.
Answer:
Kinetic energy of the electron, Ek = 120 eV
Planck’s constant, h = 6.63 x 10-34 Js
Mass of an electron, m = 9.1 x 10-31
Charge on an electron, e = 1.6 x 10-19 C
(a) For the electron, we can write the relation for kinetic energy as
Ek = \(\frac{1}{2}\) mv2
where, v = speed of the electron
∴ v2 = \(\sqrt{\frac{2 e E_{k}}{m}}=\sqrt{\frac{2 \times 1.6 \times 10^{-19} \times 120}{9.1 \times 10^{-31}}}\)
= \(\sqrt{42.198 \times 10^{12}}\) = 6.496 x 106 m/s
Momentum of the electron, p = mv = 9.1 x 10-31 x 6.496 x 106
=5.91 x 1024 kg ms-1
Therefore, the momentum of the electron is 5.91 x 1024 kg ms-1.
Question 14.
The wavelength of light from the spectral emission line of sodium is 589 nm. Find the kinetic energy at which an electron, and a neutron, would have the same de Broglie wavelength.
Answer:
Wavelength of light of sodium line, λ = 589 nm = 589 x 10-9 m
Mass of an electron, me = 9.1 x 10-31 kg
Mass of a neutron, mn = 1.66 x 10-27 kg
Planck’s constant, h = 6.63 x 10-34 Js
(a) For the kinetic energy K, of an electron accelerating with a velocity v, we have the relation
K = \(\frac{1}{2}\) mev2 ………………………… (1)
We have the relation for de Broglie wavelength as
λ = \(\frac{h}{m_{e} v}\)
∴ v2 = \(\frac{h^{2}}{\lambda^{2} m_{e}^{2}}\) ………………………… (2)
Substituting equation (2) in equation (1), we get the relation
K = \(\frac{1}{2} \frac{m_{e} h^{2}}{\lambda^{2} m_{e}^{2}}=\frac{h^{2}}{2 \lambda^{2} m_{e}}\) ………….. (3)

Hence, the kinetic energy of the electron is 6.9 x 10-25 J or 4.31 µeV.
(b) Using equation (3), we can write the relation for the kinetic energy of the neutron as = \(\frac{h^{2}}{2 \lambda^{2} m_{n}}\)
= \(\frac{\left(6.63 \times 10^{-34}\right)^{2}}{2 \times\left(589 \times 10^{-9}\right)^{2} \times 1.66 \times 10^{-27}}\)
= 3.78 x 10-28
= \(\frac{3.78 \times 10^{-28}}{1.6 \times 10^{-19}} \) = 2.36 x 10-9 eV
= 2.36 neV
Hence, the kinetic energy of the neutron is 3.78 x 10-28 J or 2.36 neV.
Question 15.
What is the de Broglie wavelength of
(a) a bullet of mass 0.040 kg travelling at the speed of 1.0 km/s,
(b) a ball of mass 0.060 kg moving at a speed of 1.0 m/s, and
(c) a dust particle of mass 1.0 x 10-9 kg drifting with a speed of 2.2 m/s?
Answer:
(a) Mass of the bullet, m = 0.040 kg
Speed of the bullet, v = 1.0 km/s = 1000 m/s
Planck’s constant, h = 6.63 x 10-34 Js
de Broglie wavelength of the bullet is given by the relation
λ = \(\frac{h}{m v}\)
= \(\frac{6.63 \times 10^{-34}}{0.040 \times 1000} \) = 1.65 x 10-35 m
(b) Mass of the ball, m = 0.060 kg
Speed of the ball, v =1.0 m/s
de Brogue wavelength of the ball is given by the relation
λ = \(\frac{h}{m v}\)
= \(\frac{6.63 \times 10^{-34}}{0.060 \times 1}\) = 1.1 x 10-32 m
(c) Mass of the dust particle, m = 1 x 10-9 kg
Speed of the dust particle, v = 2.2 m/s
de Brogue wavelength of the dust particle is given by the relation
λ = \(\frac{h}{m v}\)
= \(\frac{6.63 \times 10^{-34}}{2.2 \times 1 \times 10^{-9}}\) = 3.0 x 10-25 m.
![]()
Question 16.
An electron and a photon each have a wavelength of 1.00 run. Find
(a) their momenta,
(b) the energy of the photon, and
(c) the kinetic energy of electrons.
Answer:
Wavelength of an electron (λe) and a photon (λp),λe = λp = λ = 1 nm
= 1 x 10-9 m
Planck’s constant, h = 6.63 x 10-34 Js
(a) The momentum of an elementary particle is given by de Broglie relation
λ = \(\frac{h}{p}\)
p = \(\frac{h}{\lambda}\)
It is clear that momentum depends only on the wavelength of the particle. Since the wavelengths of an electron and a photon are equal, both have an equal momentum.
∴ p= \(\frac{6.63 \times 10^{-34}}{1 \times 10^{-9}} \) =6.63 x 10-25 kgms-1
(b) The energy of a photon is given by the relation
E= \(\frac{h c}{\lambda}\)
where, speed of light, c =3 x 108 m/s
∴ E = \(\frac{6.63 \times 10^{-34} \times 3 \times 10^{8}}{1 \times 10^{-9} \times 1.6 \times 10^{-19}}\)
= 1243.1 eV = 1.243 keV
Therefore, the energy of the photon is 1.243 keV.
(c) The kinetic energy (K) of an electron having momentum p, is given by the relation
K = \(\frac{1}{2} \frac{p^{2}}{m}\)
where, m = mass of the electron = 9.1 x 10-31 kg;
p = 6.63 x 10-25 kgm s-1
∴ K = \(\frac{1}{2} \times \frac{\left(6.63 \times 10^{-25}\right)^{2}}{9.1 \times 10^{-31}}\) = 2.415 x 10-19 J
= \(\frac{2.415 \times 10^{-19}}{1.6 \times 10^{-19}}\) = 1.51 eV
Hence, the kinetic energy of the electron is 1.51 eV.
Question 17.
(a) For what kinetic energy of a neutron will the associated de Broglie wavelength be 1.40x 10-10 m?
(b) Also, find the de Brogue wavelength of a neutron, in thermal equilibrium with matter, having an average kinetic energy of (3/2) kT at 300 K.
Answer:
(a) de Brogue wavelength of the neutron, λ =1.40 x 10-10 m
Mass of a neutron,mn =1. 66 x 10-27 kg
Planck’s constant, h = 6.63 x 10-34 Js
Kinetic energy (K) and velocity ( v) are related as
K = \( \frac{1}{2} m_{n} v^{2}\) ……………………………… (1)
de Brogue wavelength (λ) and velocity (v) are related as
λ= \(\frac{h}{m_{n} v}\) ……………………………….. (2)
Using equation (2) in equation (1), we get
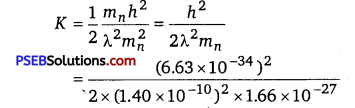
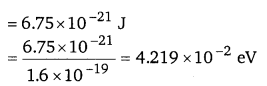
Hence, the kinetic energy of the neutron is 6.75 x 10-21 J or 4.219 x 10-2 eV.
(b) Temperature of the neutron, T = 300 K
Boltzmann’s constant, k =1.38 x 10-23 kg m2 s-2 K-1
Average kinetic energy of the neutron,
K’ = \(\frac{3}{2} \) kT.
= \(\frac{3}{2} \) x 1.38 x 10-23 x 300 = 6.21 x 10-21 J
The relation for the de Broglie wavelength is given as
λ ‘ = \(\frac{h}{\sqrt{2 K^{\prime} m_{n}}}\)
where, mn = 1.66 x 10-27 kg
h = 6.63 x 10-34 Js
K’ = 6.21 x 10-21 J
∴ λ’ = \(\frac{6.63 \times 10^{-34}}{\sqrt{2 \times 6.21 \times 10^{-21} \times 1.66 \times 10^{-27}}}\)
=1.46 x 10-10
m = 0.146 nm
Therefore, the de Broglie wavelength of the neutron is 0.146 nm.
Question 18.
Show that the wavelength cf electromagnetic radiation is equal to the de Broglie wavelength of its quantum (photon).
Answer:
The momentum of a photon having energy (hv) is given as ’
p = \(\frac{h v}{c}=\frac{h}{\lambda}\)
λ = \(\frac{h}{p}\) ………………………….. (1)
where, λ = wavelength of the electromagnetic radiation
c = speed of light
h = Planck’s constant
de Broglie wavelength of the photon is given as
λ = \(\frac{h}{m v}\)
But p = mv
∴ λ =\(\frac{h}{p}\) ………………………………….. (2)
where, m = mass of the photon
v = velocity of the photon
Hence, it can be inferred from equations (1) and (2) that the wavelength of the electromagnetic radiation is equal to the de Broglie wavelength of the photon.
Question 19.
What is the de Broglie wavelength of a nitrogen molecule in air at 300 K?
Assume that the molecule is moving with the root mean square speed of molecules at this temperature. (Atomic mass of nitrogen = 140076 u)
Answer:
Temperature of the nitrogen molecule, T = 300 K
Atomic mass of nitrogen = 14.0076 u
Hence, mass of the nitrogen molecule, m = 2 x 14.0076 = 28.0152 u
But 1 u = 1.66 x 10-27 kg
∴ m=28.0152 x 1.66 x 10-27 kg
Planck’s constant, h = 6.63 x 10-34 Js
Boltzmann’s constant, k = 1.38 x 10-23 K-1
We have the expression that relates mean kinetic energy \(\left(\frac{3}{2} k T\right)\) of the nitrogen molecuLe with the root mean square speed (vrms) as

Hence, the de Broglie wavelength of the nitrogen molecule is given as
λ = \(\frac{h}{m v_{\text {rms }}}=\frac{h}{\sqrt{3 m k T}}\)
= \(\frac{6.63 \times 10^{-34}}{\sqrt{3 \times 28.0152 \times 1.66 \times 10^{-27} \times 1.38 \times 10^{-23} \times 300}}\)
= 0.028 x 10-9 m
= 0.028 nm
Therefore, the de Broglie wavelength of the nitrogen molecule is 0.028 nm.
![]()
[Additional Exercises]
Question 20.
(a) Estimate the speed with which electrons emitted from a heated emitter of an evacuated tube impinge on the collector maintained at a potential difference of 500 V with respect to the emitter.
Ignore the small initial speeds of the electrons. The specific charge of the electron, i.e., its elm, is given to be 1.76 x 1011 Ckg-1.
(b) Use the same formula you employ in (a) to obtain electron speed for an collector potential of 10 MV. Do you see what is wrong? In what way is the formula to be modified?
Answer:
(a) Potential difference across the evacuated tube, V = 500 V
Specific charge of the electron, e/m = 1.76 x 1011 C kg-1
The speed of each emitted electron is given by the relation for kinetic energy as
KE = \(\frac{1}{2}\) mv2
Therefore, the speed of each emitted electron is
KE =\(\frac{1}{2}\) mv2 = eV
∴ v = \(\left(\frac{2 e V}{m}\right)^{1 / 2}=\left(2 V \times \frac{e}{m}\right)^{1 / 2} \)
= (2x 500 xl.76 x 1011)1/2
= 1.327 x 107 m/s
(b) Potential of the anode, V = 10 MV = 10 x 106 = 107 V
The speed of each electron is given as
v = \(\left(2 V \frac{e}{m}\right)^{1 / 2}\)
= (2 x 107x 1.76 x 1011)1/2
= 1.88 x 109 m/s .
This result is wrong because nothing can move faster than light. In the above formula, the expression (mv2 / 2) for energy can only be used in the non-relativistic limit, i. e., for v < < c.
For very high speed problems, relativistic equations must be considered for solving them. In the relativistic limit, the total energy is given as E = mc2
where, m = relativistic mass
m0 = \(\left(1-\frac{v^{2}}{c^{2}}\right)^{1 / 2}\) = mass of the particle at rest
Kinetic energy is given as
K = mc2 – m0c2
Question 21.
(a) A monoenergetic electron beam with electron speed of 5.20x 106 ms-1 is subject to a magnetic field of 1.30 x 10 4T normal to the beam velocity. What is the radius of the circle traced by the beam, given elm for electron equals 1.76x 1011 C kg-1
(b) Is the formula you employ in (a) valid for calculating radius of the path of a 20 MeV electron beam? If not, in what way is it modified? [Note: Exercises 11.20 (b) and 11.21 (b) take you to relativistic mechanics which is beyond the scope of this book. They have been inserted here simply to emphasise the point that the formulas you use in part (a) of the exercises are not valid at very high speeds or energies. See answers at the end to know what ‘very high speed or energy’ means.]
Answer:
(a) Speed of the electron, v = 5.20 x 106 m/s
Magnetic field experienced by the electron, B = 1.30 x 10-4 T
Specific charge of the electron, e/m = 1.76 x 1011 C kg’
where, e = charge on the electron = 1.6 x 10-19 C
m = mass of the electron = 9.1 x 10-31 kg
The force exerted on the electron is given as
F = e\(|\vec{v} \times \vec{B}|\)
= evBsinθ
θ = angle between the magnetic field and the beam velocity.
The magnetic field is normal to the direction of beam.
∴ θ = 90°
F = evB ……………………………. (1)
The beam traces a circular path of radius, r. It is the magnetic field, due to its bending nature, that provides the centripetal force \(\left(F=\frac{m v^{2}}{r}\right)\) for the
beam.
Hence, equation (1) reduces to
evB = \(\frac{m v^{2}}{r}\)
∴ r = \(\frac{m v}{e B}=\frac{v}{\left(\frac{e}{m}\right) B}=\frac{5.20 \times 10^{6}}{\left(1.76 \times 10^{11}\right) \times 1.30 \times 10^{-4}}\)
= 0.227 m
= 0.227 x 100 cm = 22.7 cm
Therefore, the radius of the circular path is 22.7 cm.
(b) Energy of the electron beam, E = 20 MeV = 20 x 106 x 1.6 x 10-19 J
The energy of the electron is given as
E = \(\frac{1}{2} \) mv2
∴ v = \(\left(\frac{2 E}{m}\right)^{1 / 2}\)
= \(\sqrt{\frac{2 \times 20 \times 10^{6} \times 1.6 \times 10^{-19}}{9.1 \times 10^{-31}}}\) = 2.652 x 109 m/s
This result is incorrect because nothing can move faster than light. In the above formula, the expression (mv2 / 2) for energy can only be used in the non-relativistic limit, i. e., for v« c.
When very high speeds are concerned, the relativistic domain comes into consideration. In the relativistic domain, mass is given as
m = m0 \(\left[1-\frac{v^{2}}{c^{2}}\right]^{1 / 2}\)
where, m0 = mass of the particle at rest
Hence, the radius of the circular path is given as
r = mv/eB = \(\frac{m_{0} v}{e B \sqrt{\frac{c^{2}-v^{2}}{c^{2}}}}\).
Question 22.
An electron gun with its collector at a potential of 100 V fires out electrons in a spherical hulh containing hydrogen gas at low pressure (~ 10-2 nun of Hg). A magnetic field of 2.83 x 10-4 T curves the path of the electrons in a circular orbit of radius 12.0 cm. (The path can be viewed because the gas ions in the path focus the beam by attracting electrons, and emitting light by electron capture; this method is known as the ‘fine beam tube’ method). Determine elm from the data.
Answer:
Potential of the anode, V = 100 V
Magnetic field experienced by the electrons, B = 2.83 x 10,sup>-4 T
Radius of the circular orbit r = 12.0 cm = 12.0 x 10-2 m
Mass of each electron = m
Charge on each electron = e ‘
Velocity of each electron = v
The energy of each electron is equal to its kinetic energy, i. e.,
\(\frac{1}{2}\) mv2 = eV
v2 = \(\frac{2 e V}{m}\)
It is the magnetic field, due to its bending nature, that provides the \(\left(F=\frac{m v^{2}}{r}\right) \) for the beam.
Hence, we can write Centripetal force = Magnetic force mv2
\(\frac{m v^{2}}{r}\) = evB
eB = \(\frac{m v}{r}\)
v = \(\frac{e B r}{m}\) ………………………………. (2)
Putting the value of y in equation (1), we get

Therefore, the specific charge ratio (e/ m) is 1.73 x 1011 C kg-1.
Question 23.
(a) An X-ray tube produces a continuous spectrum of radiation with its short wavelength end at 0.45 A. What is the maximum energy of a photon in the radiation?
(b) From your answer to (a), guess what order of accelerating voltage (for electrons) is required in such a tube?
Answer:
(a) Wavelength produced by the X-ray tube, λ= 0.45Å= 0.45 x 10-10 m
Planck’s constant, h = 6.63 x 10-34 Js
Speed of light, c = 3 x 108 m/s
The maximum energy of a photon is given as
E = \(=\frac{h c}{\lambda}\frac{6.63 \times 10^{-34} \times 3 \times 10^{8}}{0.45 \times 10^{-10} \times 1.6 \times 10^{19}} \) = 27.6 x 103 eV = 27.6 keV
Therefore, the maximum energy of the X-ray photon is 27.6 keV.
(b) Accelerating voltage provides energy to the electrons for producing X-rays.
To get an X-ray of 27.6 keV, the incident electrons must possess at least 27.6 keV of kinetic electric energy. Hence, an accelerating voltage of the order of 30 keV is required for producing X-rays.
![]()
Question 24.
In an accelerator experiment on high-energy collisions of electrons with positrons, a certain event is interpreted as an annihilation of an electron-positron pair of total energy 10.2 BeV into two y-rays of equal energy. What is the wavelength associated with each γ-ray? (1 BeV = 109 eV)
Answer:
Total energy of two γ-rays
E = 10.2 BeV
= 10.2 x 109 eV
= 10.2 x 109 x 1.6 x 10-19 J
= 10.2 x 1.6 x 10-10
Hence, the energy of each γ-ray
E’ = \(\frac{E}{2}\)
= \(\frac{10.2 \times 1.6 \times 10^{-10}}{2}\)
= 8.16 x 10-10 J
Planck’s constant, h = 6.63 x 10-34 Js
Speed of light, c = 3 x 108 m/s
Energy is related to wavelength as
E ‘ = \(\frac{h c}{\lambda}\)
∴ λ = \(\frac{h c}{E^{\prime}}=\frac{6.63 \times 10^{-34} \times 3 \times 10^{8}}{8.16 \times 10^{-10}}\)
= 2.436 x 10-16 m
Therefore, the wavelength associated with each γ-ray is 2.436 x 10-16 m.
Question 25.
Estimating the following two numbers should be interesting. The first number will tell you why radio engineers do not need to worry much about photons. The second number tells you why our eye can never ‘count photons’, even in barely detectable light.
(a) The number of photons emitted per second by a Mediumwave transmitter of 10 kW power, emitting radio waves of wavelength 500 m.
(b) The number of photons entering the pupil of our eye per second corresponding to the minimum intensity of white light that we humans can perceive (~ 10-10 W m-2). Take the area of the pupil to be about 0.4 cm2, and the average frequency of white light to be about 6 x 1014 Hz.
Answer:
(a) Power of the medium wave transmitter,
P = 10kW = 104 W = 104 J/s
Hence, the energy emitted by the transmitter per second, E = 104
The wavelength of the radio wave, λ = 500 m
The energy of the wave is given as E1 = \(\frac{h c}{\lambda}\)
where, h = Planck’s constant = 6.63 x 10-34 Js
c = speed of light = 3 x 108 m/s
∴ E1 = \(\frac{6.63 \times 10^{-34} \times 3 \times 10^{8}}{500}\) = 3.96 x 10-28
Let n be the number of photons emitted by the transmitter.
∴ nE1 = E
n = \(\frac{E}{E_{1}}\) =\(\frac{10^{4}}{3.96 \times 10^{-28}}\) = 2.525 x 1031
≈ 3 x 1031
The energy (E1) of a radio photon is very less, but the number of photons (n) emitted per second in a radio wave is very large. The existence of a minimum quantum of energy can be ignored and the total energy of a radio wave can be treated as being continuous.
(b) Intensity of light perceived by the human eye, I = 10-10 W m-2
Area of the pupil, A = 0.4 cm2 = 0.4 x 10-4 m2
Frequency of white light, v = 6 x 1014 Hz
The energy emitted by a photon is given as
E = hv
where, h = Planck’s constant = 6.63 x 10-34 Js
∴ E = 6.63 x 10-34 x 6 x 1014
= 3.96 x 10-19 J
Let n be the total number of photons falling per second, per unit area of the pupil. The total energy per unit for n falling photons is given as
E = n x 3.96 x 10-19 Js-1 m-2
The energy per unit area per second is the intensity of light.
∴ E = I
n x 3.96 x 10-19 = 10-10
n = \(\frac{10^{-10}}{3.96 \times 10^{-19}}\)
= 2.52 x 108 m2 s-1
The total number of photons entering the pupil per second is given as
nA =n x A
= 2.52 x 108 x 0.4 x 10-4
= 1.008 x 104 s-1
This number is not as large as the one found in problem (a), but it is large enough for the human eye to never see the individual photons.
Question 26.
Ultraviolet light of wavelength 2271 Å from a 100 W mercury source irradiates a photo-cell made of molybdenum metal. If the stopping potential is -1.3 V, estimate the work function of the metal. How would the photo-cell respond to a high intensity (~105 Wm-2) red light of wavelength 6328 Å produced by a He-Ne laser?
Answer:
Wavelength of ultraviolet light, λ = 2271 Å = 2271 x 10-10 m
Stopping potential of the metal, V0 = 1.3 V
Planck’s constant, h = 6.63 x 10-34 Js
Charge on an electron, e = 1.6 x 10-19 C
Work function of the metal = Φ0
Frequency of light = v
We have the photo-energy relation from the photoelectric effect as
Φ0 =hv0
v0 = \(\frac{\phi_{0}}{h}\)
= \(\frac{6.64 \times 10^{-19}}{6.63 \times 10^{-34}} \)
= 1.006 x 1015 Hz = 4.15 eV
Let v0 be the threshold frequency of the metal.
∴ Φ0 = hv0
v0 = \(\frac{\phi_{0}}{h}\)
= \(\frac{6.64 \times 10^{-19}}{6.63 \times 10^{-34}}\)
= 1.006 x 1015 Hz
Wavelength of red light, λr = 6328 Å = 6328 x 10-10
∴ Frequency of red light, vr = \(\frac{c}{\lambda_{r}}=\frac{3 \times 10^{8}}{6328 \times 10^{-10}} \)
= 4.74 x 1014 Hz
Since v0 > vr, the photocell will not respond to the red light produced by the laser.
![]()
Question 27.
Monochromatic radiation of wavelength 640.2 nm (1 nm = 10-9 m) from a neon lamp irradiates photosensitive material made of caesium on tungsten. The stopping voltage is measured to be 0.54 V. The source is replaced by an iron source and its 427.2 nm line irradiates the same photo-cell. Predict the new stopping voltage.
Answer:
Wavelength of the monochromatic radiation, λ = 640.2 nm = 640.2 x 10-9 m
Stopping potential of the neon lamp, V0 = 0.54 V
Charge on an electron, e = 1.6 x 10-19 C
Planck’s constant, h = 6.63 x 10-34 Js
Let Φ0 the work function and v be the frequency of emitted light. We have the photo-energy relation from the photoelectric effect as
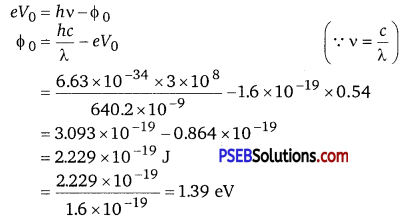
Wavelength of the radiation emitted from an iron source, λ’ = 427.2 nm = 427.2 x 10-9 m
Let V’0 be the new stopping potential. Hence, photo-energy is given as
eV’0 = \(\frac{h c}{\lambda^{\prime}}-\phi_{0}\)

Hence, the new stopping potential is 1.50 eV.
Question 28.
A mercury lamp is a convenient source for studying frequency dependence of photoelectric emission, since it gives a number of spectral lines ranging from the UV to the red end of the visible spectrum. In our experiment with rubidium photo-cell, the following lines from a mercury source were used :
λ1 =3650 Å,
λ2 = 4047 Å,
λ3 =4358 Å,
λ4 =5461 Å,
λ5 =6907 Å,
The stopping voltages, respectively, were measured to be :
V01 = 1.28 V,
V02 = 0.95 V,
V03 = 0.74 V,
V04 = 0.16 V,
V05 = 0 V.
Determine the value of Planck’s constant h, the threshold frequency and work function for the material.
[Note: You will notice that to get h from the data, you will need to know e (which you can take to be 1.6 x 10-19 C). Experiments of this kind on Na, Li, K, etc. were performed by Millikan, who, using his own value of e (from the oil-drop experiment) confirmed Einstein’s photoelectric equation and at the same time gave an independent estimate of the value of h.]
Answer:
Given, the following wavelength from a mercury source were used
λ1 =3650 Å, = 3650 x 10-10 m
λ2 = 4047 Å, = 4047 x 10-10m
λ3 =4358 Å, = 4358 x 10-10 m
λ4 =5461 Å, = 5461 x 10-10 m
λ5 =6907 Å, = 6907 x 10-10m
The stopping voltages are as follows
V01 =1.28 V,
V02 = 0.95V,
V03 =0.74V,
V04 =0.16 V,
V5 =0
Frequencies corresponding to wavelengths

As we know that
eV0 = hv-Φ0
v0 = \(\frac{h v}{e}-\frac{\phi_{0}}{e}\)
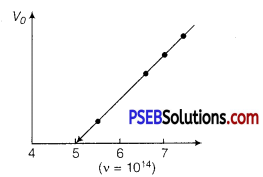
As the graph between V0 and frequency v is a straight line.
The slope of this graph gives the values of \(\frac{h}{e} \)
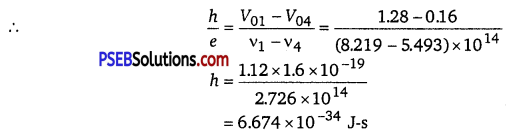
(b) Work function, Φ0 = hv0
= 6.574 x 10-34 x 5 x 1014
= 32.870 x 10-20J
= 2.05eV.
![]()
Question 29.
The work function for the following metals is given: Na: 2.75 eV; K: 2.30 eV; Mo: 4.17 eV; Ni: 5.15 eV.
Which of these metals will not give photoelectric emission for a radiation of wavelength 3300 Å from a He-Cd laser placed 1 m away from the photo-cell? What happens if the laser is brought nearer and placed 50 cm away?
Answer:
Mo and Ni will not show photoelectric emission in both cases.
Wavelength for the radiation, λ – 3300 Å = 3300 x 10-10 m
Speed of light, c = 3 x 108 m/s
Planck’s constant, h = 6.63 x 10-34 Js
The energy of incident radiation is given as
E = \(\frac{h c}{\lambda}\)
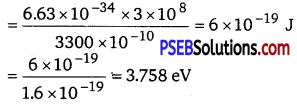
= 3.758eV
It can be observed that the energy of the incident radiation is greater than the work function of Na and K only. It is less for Mo and Ni. Hence, Mo and Ni will not show photoelectric emission. If the source of light is brought near the photocells and placed 50 cm away from them, then the intensity of radiation will increase. This does not affect the energy of the radiation. Hence, the result will be the same as before. However, the photoelectrons emitted from Na and K will increase in proportion to intensity.
Question 30.
Light of intensity 10-5 W m-2 falls on a sodium photo-cell of surface area 2 cm2. Assuming that the top 5 layers of sodium absorb the incident energy, estimate time required for photoelectric emission in the wave-picture of radiation. The work function for the metal is given to be about 2 eV. What is the implication of your answer?
Answer:
Given, intensity of light = 10-5 W/m2
Area = 2 cm2 = 2 x 10-4 m2
Work function for the metal Φ0 = 2 eV
Let t be the time.
The effective atomic area of Na = 10-20 m2 and it contains one conduction electron per atom.
Number of conduction electrons in five layers
= \(\frac{5 \times \text { Area of one layer }}{\text { Effective atomic area }}=\frac{5 \times 2 \times 10^{-4}}{10^{-20}}\)
= 107
We know that sodium has one free electron (or conduction electron) per atom.
Incident power on the surface area of photocell
= Incident intensity x Area on the surface area of photocell
= 10-5 x 2 x 10-4
= 2 x 10-9W
The electron present in all the 5 layers of sodium will share the incident energy equally.
Energy absorbed per second per electron,
E = \(\frac{\text { Incident power }}{\text { Number of electrons in five layers }} \)
= \(\frac{2 \times 10^{-9}}{10^{17}}\) = 2 x 10-26W
Time required for emission by each electron, t = \(\frac{\text { Energy required per electron }}{\text { Energy absorbed per second }} \) = \(\frac{2 \times 1.6 \times 10^{-19}}{2 \times 10^{-26}} \) = 1.6 x 107s
which is about 0.5 yr.
The answer obtained implies that the time of emission of electrons is very large and is not agreement with the observed time of emission. There is no time lag between the incidence of light and the emission of photoelectrons. Thus, it is implied that the wave theory cannot be applied in this experiment.
Question 31.
Crystal diffraction experiments can be performed using X-rays, or electrons accelerated through appropriate voltage. Which probe has greater energy? (For quantitative comparison, take the wavelength of the probe equal to 1 Å, which is of the order of interatomic spacing in the lattice) (me = 9.11 x 10-31 kg).
Answer:
An X-ray probe has a greater energy than an electron probe for the same wavelength.
Wavelength of light emitted from the probe, λ = 1 Å = 10-10 m
Mass of an electron, me = 9.11 x 10-31 kg
Planck’s constant, h = 6.63 x 10-34 Js
Charge on an electron, e = 1.6 x 10-19 C
The kinetic energy of the electron is given as
K = \(\frac{1}{2} m_{n} v^{2}\)
mnv = \(\sqrt{2 K m_{n}}\)
where, v = velocity of the electron
mnv = momentum (p) of the electron
According to the de Brogue principle, the de Brogue wavelength is given as
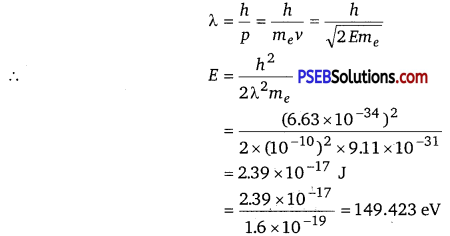
The energy of a photon,
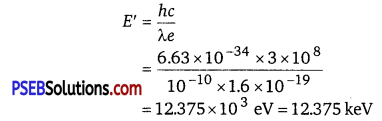
Hence, a photon has a greater energy than an electron for the same wavelength.
Question 32.
(a) Obtain the de Broglie wavelength of a neutron of kinetic energy 150 eV. As you have seen in Exercise 11.31, an electron beam of this energy is suitable for crystal diffraction experiments. Would a neutron beam of the same energy be equally suitable? Explain. (m = 1.675x 10-27 kg)
(b) Obtain the de Brogue wavelength associated with thermal neutrons at room temperature (27C). Hence, explain why a fast neutron beam needs to be thermalised with the environment before it can be used for neutron diffraction experiments.
Answer:
(a) de Brogue wavelength= 2.327 x 10-12 m; neutron is not suitable for
the diffraction experiment Kinetic energy of the neutron, K = 150 eV
= 150 x 1.6 X 10-19
=2.4 x 10-17 J
Mass of a neutron, mn = l.675 x 10-27 kg
The kinetic energy of the neutron is given by the relation
K = \(\frac{1}{2} m_{n} v^{2}\)
mnv = \(\sqrt{2 K m_{n}}\)
where, y = velocity of the neutron
mnv = momentum of the neutron
deBroglie wavelength of the neutron is given as
λ = \(\frac{h}{m_{n} v}=\frac{h}{\sqrt{2 K m_{n}}}\)
It is clear that wavelength is inversely proportional to the square root of mass.
Hence, wavelength decreases with increase in mass and vice versa.
∴ λ = \(\frac{6.63 \times 10^{-34}}{\sqrt{2 \times 2.4 \times 10^{-17} \times 1.675 \times 10^{-27}}}\)
= 2.327 x 10-12 m
It is given in the previous problem that the interatomic spacing of a crystal is about 1 Å, i.e., 10-10 m.
Hence, the interatomic spacing is about a hundred times greater. Hence, a neutron beam of energy 150 eV is not
suitable for diffraction experiments.
(b) de Brogue wavelength =1.447 x 10-10 m
Room temperature, T = 27°C = 27+ 273 = 300 K
The average kinetic energy of the neutron is given as
E=\(\frac{3}{2}\) kT
where, k = Boltzmann’s constant = 1.38 x10-23 J mol-1K-1
The wavelength of the neutron is given as
λ = \(\frac{h}{\sqrt{2 m_{n} E}}=\frac{h}{\sqrt{3 m_{n} k T}}\)
= \(\frac{6.63 \times 10^{-34}}{\sqrt{3 \times 1.675 \times 10^{-27} \times 1.38 \times 10^{-23} \times 300}}\)
=1.447 x 10-10 m
This wavelength is comparable to the interatomic spacing of a crystal. Hence, the high energy neutron beam should first be thermalised, before using it for diffraction.
Question 33.
An electron microscope uses electrons accelerated by a voltage of 50 kV. Determine the de Brogue wavelength associated with the electrons, if other factors (such as numerical aperture, etc.) are taken to be roughly the same, how does the resolving power of an electron microscope compare with that of an optical microscope which uses yellow light?
Answer:
Electrons are accelerated by a voltage, V = 50 kV = 50 x 103 V
Charge on an electron, e 1.6 x 10-19 C
Mass of an electron, me = 9.11 x 10-31 kg
Wavelength of yellow light = 5.9 x 10-7 m
The kinetic energy of the electron is given as
E=eV
=l.6 x 10-19x 50x 103
= 8 x 10-15 J
de Brogue wavelength is given by the relation
λ = \(\frac{h}{\sqrt{2 m_{e} E}}\)
= \(\frac{6.63 \times 10^{-34}}{\sqrt{2 \times 9.11 \times 10^{-31} \times 8 \times 10^{-15}}}\)
= 5.467 x 10-12 m
This wavelength is nearly 105 times less than the wavelength of yellow light. The resolving power of a microscope is inversely proportional to the wavelength of light used. Thus, the resolving power of an electron microscope is nearly 105 times that of an optical microscope.
![]()
Question 34.
The wavelength of a probe is roughly a measure of the size of a structure that it can probe in some detail. The quark structure of protons and neutrons appears at the minute length scale of 10-15 m or less. This structure was first, probed in early 1970s using high energy electron beams produced by a linear accelerator at Stanford, USA. Guess what might have been the order of energy of these electron beams. (Rest mass energy of electron = 0.511 MeV.)
Answer:
Wavelength of a proton or a neutron, λ ≈ 10-15 m
Rest mass energy of an electron,
m0c2 =0.511 MeV
= 0.511 x 106 x 1.6 x 10-19
= 0.8176 x 10-13 J
Planck’s constant, h = 6.63 x 10-34 Js
Speed of light, c = 3 x 108 m/s ,
The momentum of a proton or a neutron is given as
p = \(\frac{h}{\lambda}\)
= \(\frac{6.63 \times 10^{-34}}{10^{-15}}\) = 6.6 x 10-19 kg m/s
The relativistic relation for energy (E) is given as
E2 = p2c2 +m02c4
= (6.6x 10-19 x 3 x 108)2 + (0.8176 x 10-13)2
= 392.04 x 10-22 +0.6685 x 10-26
≈ 392.04 x 10-22
∴ E = 1.98x 10-10 J
Thus, the electron energy emitted from the accelerator at Stanford, USA might be of the order of 1.24 BeV.
Question 35.
Find the typical de Broglie wavelength associated with a He atom in helium gas at room temperature (27°C) and 1 atm pressure, and compare it with the mean separation between two atoms under these conditions.
Answer:
de Broglie wavelength associated with He atom = 0.7268 x 10-10 m .
Room temperature, T = 27°C =27 + 273 = 300 K
Atmospheric pressure, P = 1 atm = 1.01 x 105 Pa
Atomic weight of a He atom = 4
Avogadro’s number, NA = 6.023 x 1023
Boltzmann’s constant, k = 1.38 x 10-23 J mol-1 K-1
Average energy of a gas at temperature T, is given as
E = \(\frac{3}{2}\) kT
de Broglie wavelength is given by the relation
λ = \(\frac{h}{\sqrt{2 m E}}\)
where, m = mass of a He atom
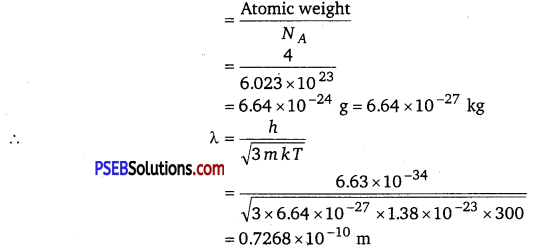
We have the ideal gas formula
PV = RT
PV = kNT
∵ \(\frac{V}{N}=\frac{k T}{P}\)
where V = volume of the gas
N = number of moles of the gas
Mean separation between two atoms of the gas is given by the relation
r = \(\left(\frac{V}{N}\right)^{1 / 3}=\left(\frac{k T}{P}\right)^{1 / 3}=\left[\frac{1.38 \times 10^{-23} \times 300}{1.01 \times 10^{5}}\right]^{1 / 3}\)
= 3.35 x 10-9 m
Hence, the mean separation between the atoms is much greater than the de Broglie wavelength.
Question 36.
Compute the typical de Broglie wavelength of an electron in a metal at 2 7° C and compare it with the mean separation between two electrons in a metal which is given to be about 2 x 1010 m. [Note: Exercises 11.35 and 11.36 reveal that while the wave-packets associated with gaseous molecules under ordinary conditions are non-overlapping, the electron wave- packets in a metal strongly overlap with one another. This suggests that whereas molecules in an ordinary gas can be distinguished apart, electrons in a metal cannot be distinguished apart from one another. This indistinguishability has many fundamental implications which you will explore in more advanced Physics courses.]
Answer:
Temperature, T = 27°C = 27 +273 = 300 K
Mean separation between two electrons, r = 2 x 10-10 m
de Broglie wavelength of an electron is given as
λ = \(\frac{h}{\sqrt{3 m k T}}\)
where,
h = Planck’s constant = 6.63 x 10-34 Js
m = Mass of an electron = 9.11 x 10 -31 kg
k = Boltzmann’s constant = 1.38 x 10-23 J mol-1 K-1
∴ λ = \(\frac{6.63 \times 10^{-34}}{\sqrt{3 \times 9.11 \times 10^{-31} \times 1.38 \times 10^{-23} \times 300}}\)
= 6.2 x 109 m
Hence, the de Broglie wavelength is much greater than the given inter-electron separation.
Question 37.
Answer the following questions :
(a) Quarks inside protons and neutrons are thought to carry fractional charges [(+2/3)e ; (-1/3)e]. Why do they not show up in Millikan’s oil-drop experiment?
(b) What is so special about the combination e/m? Why do we not simply talk of e and m separately?
(c) Why should gases be insulators at ordinary pressures and start conducting at very low pressures?
(d) Every metal has a definite work function. Why do all photoelectrons not come out with the same energy if incident radiation is monochromatic? Why is there an energy distribution of photoelectrons?
(e) The energy and momentum of an electron are related to the frequency and wavelength of the associated matter wave by the relations :
E = hv, p = \(\frac{\boldsymbol{h}}{\lambda}\)
But while the value of λ is physically significant, the value of v (and therefore, the value of the phase speed vλ) has no physical significance. Why?
Answer:
(a) Quarks inside protons and neutrons carry fractional charges. This is because nuclear force increases extremely if they are pulled apart. Therefore, fractional charges may exist in nature; observable charges are siti the integral multiple of an electrical charge.
(b) Thè basic relations for electric field and magnetic field are \(\left(e V=\frac{1}{2} m v^{2}\right)\) and \(\left(e B v=\frac{m v^{2}}{r}\right) \) respectively.
These relations include e (electric charge), y (velocity), m (mass), V (potential), r (radius), and B (magnetic field)._These relations give the value of velocity of an electron as \(\left(v=\sqrt{2 v\left(\frac{e}{m}\right)}\right) \text { and }\left(v=B r\left(\frac{e}{m}\right)\right)\) respectively. It can be observed from these relations that the dynamics of an electron is determined not by e and m separately, but by the ratio e / m.
(c) At atmospheric pressure, the ions of gases have no chance of reaching their respective electrons because of collision and recombination with other gas molecules. Hence, gases are insulators at atmospheric pressure. At low pressure, ions have a chance of reaching their respective electrodes and constitute a current. Hence, they conduct electricity at these pressures.
(d) The work function of a metal is the minimum energy required for a conduction electron to get out of the metal surface. All the electrons in an atom do not have the same energy level. When a ray having some photon energy is incident on a metal surface, the electrons come out from different levels with different energies. Hence, these emitted electrons show different energy distributions.
![]()
(e) The absolute value of energy of a particle is arbitrary within the additive constant. Hence, wavelength (λ) is significant, but the frequency (v) associated with an electron has no direct physical significance. Therefore, the product vλ (phase speed) has no physical significance. Group speed is given as
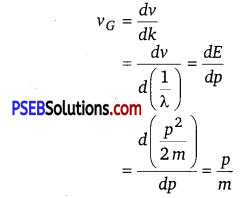
This Quantity has a physical meaning.
