Punjab State Board PSEB 7th Class Science Book Solutions Chapter 5 Acids, Bases and Salts Textbook Exercise Questions and Answers.
PSEB Solutions for Class 7 Science Chapter 5 Acids, Bases and Salts
Science Guide for Class 7 PSEB Acids, Bases and Salts Intext Questions and Answers
Think and Answer (Textbook Page No. 54)
Question 1.
What will be the colour of basic solution after the addition of pehnolphthalein?
Answer:
Adding phenolphthalein to the alkaline solution turns its colour to pink.
Question 2.
Name the products of neutralisation.
Answer:
In the process of neutralisation salt and water are produced in the form of products.
PSEB 7th Class Science Guide Acids, Bases and Salts Textbook Questions and Answers
1. Fill in the blanks:
(i) Acids are …………….. in taste.
Answer:
sour
![]()
(ii) Litmus and turmeric extract are …………….. indicators.
Answer:
natural
(iii) Phenolphthalein is ………………………… in acidic solution.
Answer:
pink
(iv) Reaction between an acid and a …………………… is called neutralisation reaction.
Answer:
alkali (Base)
(v) Ant’s sting has ………………….. acid.
Answer:
formic
(vi) Excess secretion of hydrochloric acid in stomach, is called ……………………….. .
Answer:
indigestion
(vii) Milk of magnesia is used in case of ……………………. .
Answer:
acidity
2. Match the Column ‘A’ with Column ‘B’:
| Column ‘A’ | Column ‘B’ |
| 1. Red litmus changes to blue in | (a) Neutralisation |
| 2. Blue litmus changes to red in | (b) Zinc Carbonate |
| 3. Reaction between acid and a base | (c) Basic solution |
| 4. Formic acid | (d) Antbite |
| 5. Calamine | (e) Acidic Solution |
Answer:
| Column ‘A’ | Column ‘B’ |
| 1. Red litmus changes to blue in | (c) Basic solution |
| 2. Blue litmus changes to red in | (e) Acidic Solution |
| 3. Reaction between acid and a base | (a) Neutralisation |
| 4. Formic acid | (d) Antbite |
| 5. Calamine | (b) Zinc Carbonate |
![]()
3. Choose the Correct Answer:
Question (i)
Vinegar contains :
(a) acetic acid
(c) citric acid
Ans.
(a) acetic acid .
Question (ii)
Tamarind contains :
(a) acetic acid
(b) lactic acid
(c) citric acid
(d) tartaric acid
Answer:
(d) tartaric acid
Question (iii)
The example of natural indicator is
(a) Litmus
(b) Turmeric extract
(c) China rose petals
(d) All the above
Answer:
(d) All the above
Question (iv)
The colour of blue litmus in acidic solution in :
(a) purple
(b) blue
(c) red
(d) pink
Answer:
(c) red
Question (v)
Amla contains :
(a) ascorbic acid
(b) quick lime
(c) calmine
(d) All the above
Answer:
(a) Ascorbic acid
4. Write True or False:
(i) Citric acid is found in tamarind.
Answer:
False
(ii) Ant’s sting has oxalic acid.
Answer:
False
(iii) Turmeric extract gives reddish brown colours in basic solution.
Answer:
True
![]()
(iv) Sodium hydroxide turns blue litmus red.
Answer:
False
(v) Organic matter is used to treat acidic soil.
Answer:
False
5. Very Short Answer Type Questions:
Question (i)
Which acid is secreted in our stomach ?
Answer:
Gastric acid is excreted in our stomach.
Question (ii)
Name any two ant acids.
Answer:
Names of two antacids :
- Magnesium Hydroxide,
- Baking Soda.
Question (iii)
What type of substances are used as ant bites ?
Answer:
Solution of calamine or baking soda is used to treat ant-stings.
Question (iv)
Name any two citric fruits.
Answer:
Names of Citrus Fruits :
- Orange,
- Lemon,
- Grapes.
Question (v)
Why is it essential to treat acidic products ?
Answer:
Factory and industry residues are naturally acidic. If thrown away directly without treatment, it can harm aquatic life. To neutralize them, some base is added to such wastes.
![]()
6. Short Answer Type Questions:
Question (i)
Name the source from which litmus solution is obtained. What is the use of this solution ?
Answer:
The solution of litmus is obtained from a plant called lichens found in nature. A strip of paper dipped in a solution of litmus is called litmus paper and the solution is called litmus solution. It is available as red and blue litmus.
Blue litmus turns red when dissolved in acidic solution and red litmus turns blue when dissolved in alkaline solution.
Question (ii)
Is the distilled water acidic/basic/neutral ? How would you verify it ?
Answer:
Distilled water is neutral. This is confirmed by the addition of litmus with which it gives green colour. The colour of Red litmus and blue litmus remain unchanged when added to distilled water showing that it is neutral in character.
Question (iii)
Describe the process of neutralisation with the help of an example.
Answer:
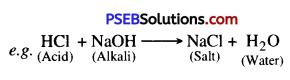
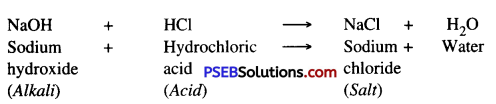
Neutralisation. The chemical reaction between an acid and an alkali is called neutralisation. As energy is released, salt and water are formed as products.
Acid + Alkali → Salt + Water + Heat (Energy)
Example : Hydrochloric Acid + Sodium Hydroxide → Sodium chloride + Water + Energy
Experiment: Fill a quarter of a test tube with dilute hydrochloric acid. Now add 2-3 drops of phenolphthalein solution (indicator) and note the color of the test tube solution. Now with the help of a dropper add a few drops of sodium hydroxide (alkali) in the test tube and gently shake the test tube. To the solution while stirring constantly, add Sodium hydroxide (alkali) solution till it turns light pink.
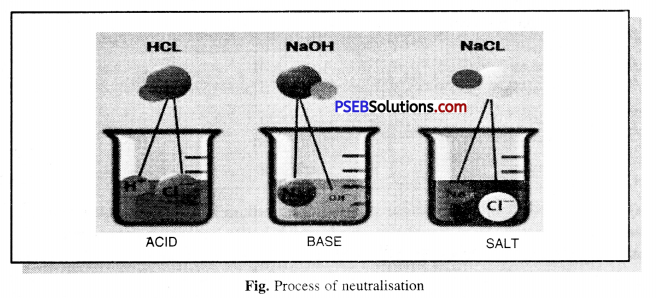
Now mix a drop of dilute Hydrochloric acid to it. You will notice that the color of the solution has disappeared (colourless) again. It is clear from this that phenolphthalein is pink in color as long as the solution is alkaline and the solution becomes colourless when the solution becomes acidic.
By mixing alkali solution with acid solution, they react with each other to neutralize the solution, i.e. the nature of acid and base gets cancelled out. This reaction is called Neutralisation.
Question (iv)
Name any two common acids and two common bases.
Answer:
Common acids. (1) Hydrochloric acid. (2) Sulphuric acid.
Common Bases. (1) Sodium hydroxide, (2) Calcium hydroxide
Question (v)
What are indicators ? Write their types and two examples of each.
Answer:
Indicators. Solution of substances that on reaction with acids, alkalis and neutral substances, give different colours, they are called indicators.
Types of indicators. There are two types of indicators:
- Natural indicators. These are indicators that are obtained from plants such as litmus, turmeric, China rose petals etc.
- Synthetic Indicators. These are indicators that are prepared in the laboratory, such as phenolphthalein and methyl orange etc.
![]()
7. Long Answer Type Questions:
Question (i)
State differences between acids and bases.
Answer:
Differences between Acids and Bases :
| Acids | Bases |
| 1. They are sour in taste.
2. They change the solution of blue litmus to red. 3. They don’t seem like soap when touched. 4. It doesn’t change colour of phenolphthalein solution. 5. They react with bases to produce salt, water and heat. |
1. They are bitter in taste.
2. They change solution of red litmus to blue color. 3. They appear like soap when touched. 4. They react with phenolphthalein solution to make it pink. 5. They react with acid to produce salt, water and heat. |
Question (ii)
Name the acid present in : (1) Vinegar (2) tamarind (3) citrus fruits and (4) curd.
Answer:
Substance The name of the acid
| 1. Vinegar | 1. Acetic acid |
| 2. Tamarind | 2. Tartaric acid |
| 3. Citric fruit | 3. Citric acid |
| 4. Spinach | 4. Oxalic acid |
| 5. Yogurt | 5. Lactic acid |
Question (iii)
You are given hydrochloric acid solution, sodium hydroxide solution and water in three different bottles. How would you check which bottle has which compound ?
Answer:
1. Take three test tubes. Take a few drops of the solution from each bottle separately in these three test tubes. Now add three drops of phenolphthalein solution to each of these test tubes. The test tube in which pink colour is observed contains base (Sodium Hydroxide) while the colour will not change in the other two test tubes.
2. Wash the test tubes and again take 5-5 drops of each solution in three different test tubes as before. Now put two drops of blue litmus in these test tubes. The test tube in which the blue litmus turns red contains acid (Hydrochloric acid).
3. Now we know that the third test tube contains water in which red and blue litmus don’t show any change.
In this way, we can find out which solution is present in which bottle.
PSEB Solutions for Class 7 Science Acids, Bases and Salts Important Questions and Answers
1. Fill in the Blanks:
(ii) is used to cure indigestion.
Answer:
(iii) Treatment of soil acidity is done by adding
Answer:
Lime
(iv) on reaction with a solution of phenolphthalein turn it pink.
Answer:
Alkali
(v) In the process of Neutralisation and are produced as a products.
Answer:
Salt, water
2. Match the Column A with Column B:
| Column ‘A’ | Column ‘B’ |
| (i) Gooseberries | (a) Acetic acid |
| (ii) Indigestion | (b) Quick lime |
| (iii) Vinegar | (c) Milk of Magnesia |
| (iv) Treatment of acidic soil | (d) Ascorbic acid |
Answer:
| Column ‘A’ | Column ‘B’ |
| (i) Gooseberries | (d) Ascorbic acid |
| (ii) Indigestion | (c) Milk of Magnesia |
| (iii) Vinegar | (a) Acetic acid |
| (iv) Treatment of acidic soil | (b) Quick lime |
![]()
3. Choose the Correct Answer:
Question (i)
Curd tastes sour so it is :
(a) basic
(b) acidic
(c) salt
(d) None of these.
Answer:
(b) acidic.
Question (ii)
Bases are :
(a) sour
(b) saltish
(c) bitter
(d) Neither sour nor sweet.
Ans.
(c) bitter.
Question (iii)
The acid present in vinegar is :
(a) Formic acid
(b) Citric acid
(c) Acetic acid
(d) Lactic acid.
Answer:
(c) Acetic acid.
Question (iv)
The acid present in curd is :
(a) Acetic acid
(b) Formic acid
(c) Citric acid
(d) Lactic acid.
Answer:
(d) Lactic acid.
(a) Calcium hydroxide
(b) turns blue litmus paper to red.
(c) Magnesium hydroxide
(a) Calcium hydroxide.
Acidic solution:
(a) turns red litmus paper to blue
(b) turns blue litmus paper to red
(c) neither turns blue litmus paper to red nor red litmus to blue
(d) None of these.
4. State True or False:
(i) Nitric acid turns red litmus blue.
Answer:
False
(ii) Sodium hydroxide turns blue litmus red.
Answer:
False
(iii) Sodium hydroxide and hydrochloric acid neutralize each other and form salt and water.
Answer:
True
(iv) Indicator is a substance which shows different colours in acidic and basic solution.
Answer:
True
![]()
(v) Tooth decay is caused by the presence of a base.
Answer:
False
Very Short Answer Type Questions
Question 1.
Name different types of substances based on their chemical nature.
Answer:
- Acidic
- Basic
- Neutral.
Question 2.
Name few substances that contain natural acids.
Answer:
Curd, lemon juice, orange juice, vinegar.
Question 3.
Name few substances which are basic in nature.
Answer:
Washing soda, baking soda.
Question 4.
Name the substance which is used to test the nature of chemical compounds.
Answer:
Indicator.
Question 5.
Name few natural indicators.
Answer:
Turmeric, litmus, China rose petals.
Question 6.
Which acid is present in curd ?
Answer:
Lactic acid.
Question 7.
What is household name of Acetic acid ?
Answer:
Vinegar.
![]()
Question 8.
Amla is rich in which acid ?
Answer:
Ascorbic acid.
Question 9.
What is lime water ?
Answer:
It is calcium hydroxide, a base.
Question 10.
Name a base found in soaps.
Answer:
Sodium hydroxide.
Question 11.
What is use of Ammonium hydroxide ?
Answer:
For cleaning window glass panes.
Question 12.
What is source of litmus ?
Answer:
Lichens.
Question 13.
What are neutral substances ?
Answer:
Neutral Substances. The substances which do not change the colour of either blue or red litmus, are neutral substances.
Short Answer Type Questions
Question 1.
Write the properties of bases.
Answer:
Properties of Bases,
- Bases are bitter to taste.
- All alkalies have a slippery touch much like that of soap.
- Bases turn red litmus paper blue.
- Bases turn phenolphthalein solution from colourless to pink.
![]()
Question 2.
Write the properties of acids.
Answer:
Properties of Acids.
- Acids turn blue litmus red.
- Acids contain hydrogen atom.
- Acids are sour in taste.
- Acids react with bases to form salt and water.
Question 3.
What is an indicator ? Name an indicator.
Answer:
Indicator. The chemicals or substances which give different colours with acids and bases, are known as acid-base indicators or simply indicators.
Phenolphthalein is another indicator which gives pink colour in alkaline solution and is colourless in acidic solution.
Question 4.
What is neutralization reaction ?
Answer:
Neutralization reaction. The process of treating an acid with an alkali/base to form a salt and water, is called neutralization reaction.
Question 5.
How the salts are formed?
Answer:
Salts. Salts are formed when an acid reacts with a base or salt is a compound which is formed by combination of acid with base.

Question 6.
Three liquids are given to you. One is hydrochloric acid, another is sodium hydroxide and third is a sugar solution. How will you identify them ? You have only turmeric powder.
Answer:
Turmeric powder is a natural indicator. With turmeric powder, turmeric strips are prepared which give different colour in three given liquids.
Question 7.
Blue litmus paper when dipped in a solution remains blue. What is the nature of the solution ? Explain.
Answer:
Acids turn blue litmus red while bases do not change the colour of blue litmus. Therefore, the given solution is base as it has not changed the colour of blue litmus paper.
Question 8.
Explain why :
(i) An antacid tablet is taken when you suffer from acidity.
(ii) Calamine solution is applied on skin when an ant bites.
(ii) Factory waste is neutralized before disposing it into the water bodies.
Answer:
(i) An antacid tablet is taken when you suffer from acidity. To neutralize acidity, antacid tablet such as milk of magnesia is taken because it contains Magnesium hydroxide (base) which neutralizes the effect of acids.
(ii) Calamine solution is applied on skin when an ant bites. Ant bite contains formic acid which gets neutralized by calamine solution (zinc carbonate).
(iii) Factory waste is neutralized before disposing it into the water bodies. Factory waste usually contains acids, so they have to be neutralized as they can kill aquatic animals and plants. Some basic substances are used to neutralize such wastes.
Long Answer Type Question
Question 9.
What are the uses of neutralization in our daily life? Explain in detail.
Answer:
Uses of Neutralisation in daily life:
(i) As Antacids.
We know that in human stomach acid is produced called as stomach acids which contain hydrochloric acid which helps in digestion of food. But too much of it can cause indigestion, abdominal pain, and heartburn, which is called acidity. To neutralize this excess acid, some mild alkali is used to relieve the pain. Such substances are called antacids, such as milk of magnesia (magnesium hydroxide), baking soda, etc.
(ii) As a treatment for insect stings.
Different species of insects such as; bees, wasps, spiders, and ants, etc. release formic acid in the body when they sting our body. The effect of formic acid can be reduced by neutralizing it with some mild alkali, such as baking soda or calamine solution.
(iii) As a treatment for soil acidity and alkalinity.
The presence of certain substances makes the soil more acidic or more alkaline. Excessive use of chemical fertilizers makes the soil acidic. Soil should be neutral for the proper growth and development of plants. Soils are tested and if it is acidic, it is treated with lime (calcium oxide), Quick lime (Calcium hydroxide), etc. But if the soil is alkaline, it is mixed with organic matter which releases acid and neutralizes the alkali present in the soil.
(iv) As a treatment for factory wastes.
Industry and factory wastes are naturally acidic. If it is thrown away directly, it can affect and harm aquatic life. Therefore, it is important to neutralize the acid present in that waste. So some alkali is added to treat it.
