Punjab State Board PSEB 8th Class Science Book Solutions Chapter 14 Chemical Effects of Electric Current Textbook Exercise Questions and Answers.
PSEB Solutions for Class 8 Science Chapter 14 Chemical Effects of Electric Current
PSEB 8th Class Science Guide Chemical Effects of Electric Current Textbook Questions and Answers
Exercises
Question 1.
Fill in the blanks.
(a) Most liquids that conduct electricity are solutions of ……………….. , ………………. and ………………. .
(b) The passage of an electric current through a solution causes ……………….. effects.
(c) If you pass current through copper sulphate solution copper gets deposited on the plate connected to the ………………… terminal of the battery.
(d) The process of depositing a layer of any desired metal on another material, by means of electricity is called ……………………… .
Answer:
(a) acid, bases, salts.
(b) chemical
(c) negative (- ve)
(d) electroplating.
Question 2.
When the free ends of a tester are dipped in a solution, the magnetic compass needle shows deflection. Can you explain the reason ?
Answer:
Deflection of compass needle is due to conduction of electricity through the solution because the solution is good conductor.
![]()
Question 3.
Name three liquids, which when tested in a manner shown in fig. may causes the magnetic needle to deflect.
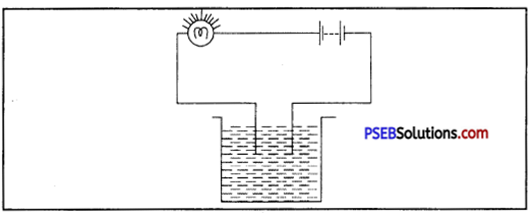
Answer:
- Acid
- Base
- Acidulated water.
Question 4.
The bulb does not glow in the setup shown in fig. List the possible reasons. Explain you answer.
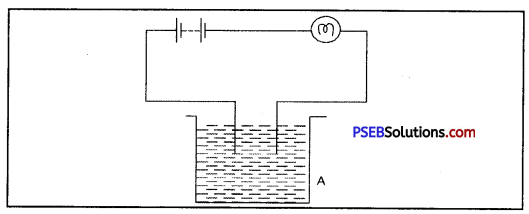
Answer:
The set up shows no glowing of the bulb but it does not mean that liquid is not conducting electricity. Liquid may be so weak electrolyte that it can not make the bulb glow. So, to test it for surity, LED can be used, which glows for very minute currents.
Question 5.
A tester is used to check the conduction of electricity through two liquids labelled A and B. It is found that the bulb of the tester glows very brightly for liquid A while it glows dimly for liquid B. You would conclude that.
(i) liquid A is better conductor than liquid B.
(ii) liquid B is better conductor than liquid A.
(iii) both liquids are equally conducting.
(iv) conducting properties of liquids cannot be compared in this manner.
Answer:
(i) Liquid A is better conductor than liquid B.
Question 6.
Does pure water conduct electricity ? If not, what can we do to make it conducting ?
Answer:
Pure water does not conduct electricity but it can be made good conductor by adding few drops of dil. sulphuric acid. The water so obtained is called acidulated water.
Question 7.
In case of a fire, before the firemen use the water hoses, they shut off the main electrical supply for the area. Explain why they do this.
Answer:
Pure water is an insulator but tap water is a good conductor of electricity. To avoid electrocuting, firemen manually shut off the supply before using water hoses.
Question 8.
A child staying in the coastal regions tests the drinking water and also the sea water with his tester. He finds that the compass needle deflects more in case of sea water. Can you explain the reason ?
Answer:
Since sea water is rich in salt concentration. So, compass needle is more deflected in sea water as compared to the drinking water available in coastal areas.
![]()
Question 9.
Is it safe for electrician to carry out electrical repairs outdoor during heavy downpour ? Explain.
Answer:
No, it is not safe for a wireman to carry out electrical repairs during heavy down pour because water (Impure) is a conductor of electricity. So, wireman can get electric shock.
Question 10.
Paheli had heard that rain water is as good as distilled water. So she collected some rainwater in a clean glass tumbler and tested it using a tester. To her surprise she found that compass needle showed deflection. What could be the reasons ?
Answer:
No doubt, rain water is pure like distilled water. But then environment is contaminated with many impurities. These impurities get dissolved in rain water, making it a conductor of electric current.
Question 11.
Prepare a list of objects around you that are electroplated.
Answer:
Electroplated objects.
- Handle bar of cycle.
- Wheel rims.
- Artificial ornaments.
- Bath taps.
- Kitchen gas burners.
Question 12.
The process that you saw in Activity 14.7 is used for purification of copper. A thin plate of pure copper and a thick rod of impure copper are used as electrodes. Copper from impure rod is sought to be transferred to the thin copper plate. Which electrode should be attached to positive terminal of the battery and why ?
Answer:
Thick rod of impure copper is to be attached to positive terminal of battery as pure copper moves to electrode connected to negative terminal of the battery.
PSEB Solutions for Class 8 Science Chemical Effects of Electric Current Important Questions and Answers
Multiple Choice Questions
Question 1.
In order to keep the flow of electricity continue in circuit, which of the following items should be used in place of iron nail in the given circuit.

(a) Graphite
(b) Plastic
(c) Wood
(d) Rubber.
Answer:
(a) Graphite.
Question 2.
Pankaj knows that pure water is bad conductor of electricity. If you dissolve salt in water then what will happen ?
(а) Water will become good conductor of electricity.
(b) Water will remain bad conductor of electricity.
(c) Water will neither be a good conductor nor a bad conductor of electricity.
(d) None of the above.
Answer:
(a) Water will become good conductor of electricity.
![]()
Question 3.
Which out of the following is insulator of electricity ?
(a) Acid
(b) Bases
(c) Pure water
(d) Solutions of salt
Answer:
(c) Pure water
Question 4.
Which out of the following is not an insulator ?
(a) Rubber
(b) Plastic
(c) Wood
(d) Copper
Answer:
(d) Copper
Question 5.
What is the effect of passing electric current through the solution of electrolytes ?
(a) Magnetic effect
(b) Heat effect
(c) Chemical effect
(d) None of these.
Answer:
(c) Chemical effect.
Question 6.
Which effect of electric current is used in electroplating ?
(a) Magnetic effect
(b) Chemical effect
(c) Heat effect
(d) None of these.
Answer:
(b) Chemical effect
Question 7.
The availability of some amount of impurities make water:
(a) conductor
(b) insulator
(c) pure
(d) none of these.
Answer:
(a) conductor.
Question 8.
A tester is used to check the conduction of electricity through two liquids labelled A and B. It is found that the bulb of the tester glows very brightly for liquid A while it glows dimly for liquid B, you would conclude that:
(а) Liquid A is better conductor than liquid B
(b) Liquid B is better conductor than liquid A
(c) Both liquids are equally conducting
(d) Conducting properties of liquids cannot be compared in this manner.
Answer:
(a) liquid A is better conductor-than liquid B.
![]()
Very Short Answer Type Questions
Question 1.
Is human body a conductor or an insulator ?
Answer:
Conductor.
Question 2.
What are conductors ?
Answer:
Conductors.
Materials like silver, copper, aluminium, iron and human body etc. which allow electric current to pass through them, are called conductors.
Question 3.
What are insulators ? Give two examples.
Answer:
Insulators.
Materials like wood, rubber, silk, plastic etc. which do not allow electric current to pass through them, are called insulators.
Question 4.
Do all liquids allow electric current to pass through them ?
Answer:
No.
Question 5.
What is LED ? ,
Answer:
LED-Light Emitting Diode.
Question 6.
Name the phenomenon of breaking up of a chemical compound under the action of electric current.
Answer:
Electrolysis.
![]()
Question 7.
What do we get on electrolysis of acidulated water ?
Answer:
Hydrogen gas and Oxygen gas.
Question 8.
Which effect of current is used in electroplating ?
Answer:
Chemical effect.
Question 9.
Which effect of current makes a bulb glow ?
Answer:
Heating effect.
Question 10.
How can small current be tested ?
Answer:
By using LEDs.
Question 11.
Name different effects of electric current.
Answer:
- Heating effect,
- lighting effect,
- chemical effect and
- magnetic effect.
Question 12.
Is air an insulator ?
Answer:
Yes.
Question 13.
Name few liquids which can conduct electricity.
Answer:
Lime water, lemon juice, vinegar, tap water.
![]()
Question 14.
Can conductors be classified as insulators or vice versa under special conditions ?
Answer:
Yes.
Question 15.
Which commonly used liquids can conduct electricity ?
Answer:
Solution of acids, bases and salts.
Question 16.
What are electrodes ?
Answer:
Electrodes.
Metallic rods or plates immersed in electrolytes to make contact with battery, are called electrodes.
Question 17.
By which phenomenon cheap articles are coated with gold or expensive metals ?
Answer:
Electroplating.
Question 18.
Is electroplating useful process ?
Answer:
Yes.
Short Answer Type Questions
Question 1.
Air is a bad conductor of electricity. Show with an experiment.
Answer:
Air-a bad conductor of electricity.
Take a battery bulb and connect it to a cell and a switch. When the switch is fixed with a safety pin then the current flows and bulb glows, but when safety pin is removed then there is only air between the gap of switch and current does not flow. It shows that air is bad conductor of electricity.
![]()
Question 2.
What is electrolysis ?
Answer:
Electrolysis.
Breaking up of chemical compounds under the action of electric current, is called electrolysis. When we pass electric current through water (acidulated), it breaks up into its constituents : hydrogen and oxygen. Hydrogen is liberated at the cathode whereas oxygen is liberated at the anode.
Question 3.
What is electroplating ?
Answer:
Electroplating.
Process of electrolysis is used to deposit thin layers of valuable metals (like zinc, silver or gold) on cheaper metals by the passage of electricity through electrolyte to save them from rusting whereas to give them a decorative look. This process is called electroplating.
Question 4.
Give a brief account of LED.
Answer:
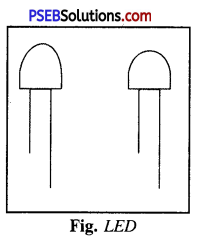
LED.
It is light emitting diode. It glows even when the current is .very small. It consists a bulb with two legs called leads. One leg is longer and other leg is shorter.
Longer leg is attached to +ve terminal of battery and shorter leg is attached to -ve terminal.
Long Answer Type Questions
Question 1.
Is water a good conductor ? What happens when common salt is added to water ?
Answer:
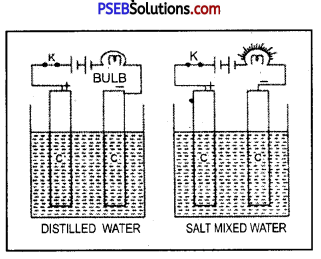
Pure or distilled water is an insulator.
Experiment.
Dip two carbon rods in distilled water and connect it to a bulb, 6 V battery and a key. The bulb will not light up showing that distilled water is a bad conductor of electricity.
Now replace distilled water by water mixed with common salt. The bulb will at once light up, when key is introduced. This shows that distilled water is perfectly insulator, impure water specially one containing common salt is highly conducting.
Question 2.
What is electroplating ? Give its uses.
Answer:
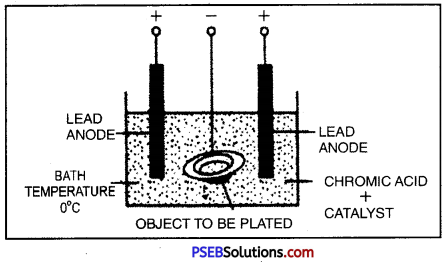
Electroplating.
It is the process by which a costlier metal is deposited on base or cheap metal by passing current through its salt solution. When an electric current is passed through an electrolyte (the compound) breaks up into its constituent ions.
Positive ions are attracted towards cathode (negative electrode) and negative ions are attracted towards anode (positive electrode). This process of electrolysis is used in plating materials with a thin coat of metals is called electroplating.
Uses of Electroplating:
- Iron is electroplated with nickel or chromium to prevent it from rusting.
- Artificial jewellery, made from cheap metals is electroplated with expensive metals like gold and silver to give it an attractive look.
- Handle bars of cycle, wheel rims, car parts etc. are coated with chromium to give a shiny look.
- Tin cans are made by electroplating a layer of tin on the iron.
Question 3.
How can a spoon be copper plated ?
Answer:
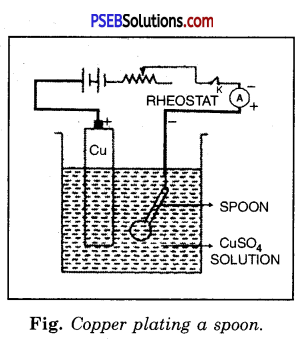
Experiment.
Take a spoon that is to be electroplated with copper. Take a copper plate and make it anode by connecting it to the positive terminal of the battery. The spoon is connected to the negative terminal of the battery. A rheostat (Variable resistance); key and ammeter are also connected in series as shown. CuSO4 solution is put in a glass vessel. A rheostat is adjusted till a proper current flows through electrolyte [For best electroplating 1A of current should be passed for every 100 cm2 of the surface to be electroplated say if the area of a spoon on both sides is 60 cm2, a current of 0.6 A should be passed.] Pass the current for 5-10 minutes, till a layer of shinning copper is seen deposited on a spoon.
