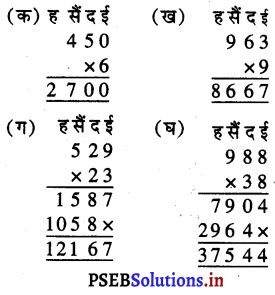
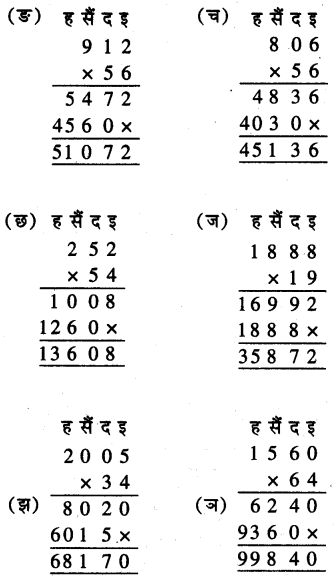
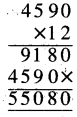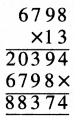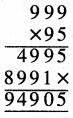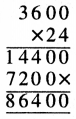Punjab State Board PSEB 5th Class Welcome Life Book Solutions Chapter 4 ਦਿਲ ਵਿੱਚ ਇਹਨਾਂ ਲਈ ਪਿਆਰ ਰੱਖੀਏ Textbook Exercise Questions and Answers.
PSEB Solutions for Class 5 Welcome Life Chapter 4 ਦਿਲ ਵਿੱਚ ਇਹਨਾਂ ਲਈ ਪਿਆਰ ਰੱਖੀਏ
Welcome Life Guide for Class 5 PSEB ਦਿਲ ਵਿੱਚ ਇਹਨਾਂ ਲਈ ਪਿਆਰ ਰੱਖੀਏ Textbook Questions and Answers
(ੳ) ਆਪਣੇ ਰਾਜ ਬਾਰੇ ਜਾਣਕਾਰੀ ਅਤੇ ਸਾਂਝਾਂ
ਰੰਗਲਾ ਸਾਡਾ ਇਹ ਪੰਜਾਬ ਬੇਲੀਓ!
ਫੁੱਲਾਂ ਵਿੱਚੋਂ ਫੁੱਲ ਜਿਉਂ ਗੁਲਾਬ ਬੇਲੀਓ !
ਰੱਖਦੇ ਨਿਰਾਸ਼ਾ ਦੂਰ ਲੋਕ ਏਥੋਂ ਦੇ,
ਨਵੇਂ-ਨਵੇਂ ਦੇਖਦੇ ਨੇ ਖ਼ਾਬ ਬੇਲੀਓ !
ਅਸੀਂ ਪੰਜਾਬ ਰਾਜ ਦੇ ਵਾਸੀ ਹਾਂ ਪੰਜਾਬ ਭਾਰਤ ਦਾ ਪ੍ਰਸਿੱਧ ਰਾਜ ਹੈ ਦੁਨੀਆ ਵਿੱਚ ਪੰਜਾਬ ਦੀ ਇੱਕ ਖ਼ਾਸ ਪਛਾਣ ਹੈ ਪੰਜਾਬ ਦਾ ਨਾਂ ਪੰਜ ਦਰਿਆਵਾਂ ਸਤਲੁਜ, ਬਿਆਸ, ਰਾਵੀ, ਚਨਾਬ ਅਤੇ ਜਿਹਲਮ ਤੋਂ ਪਿਆਹੈ 1947 ਵਿੱਚ ਭਾਰਤ ਦੀ ਵੰਡ ਹੋਣ ਕਾਰਨ ਦੋ ਦਰਿਆ ਚਨਾਬ ਅਤੇ ਜਿਹਲਮ ਪਾਕਿਸਤਾਨ ਵਿਚ ਚਲੇ ਗਏ ਅੱਜ-ਕੱਲ੍ਹ ਇੱਥੇ ਤਿੰਨ ਦਰਿਆਸਤਲੁਜ, ਬਿਆਸ ਅਤੇ ਰਾਵੀਵਗਦੇ ਹਨ


ਪੰਜਾਬ ਇੱਕ ਸਰਹੱਦੀ ਰਾਜ ਹੈ ਸਰਹੱਦੀ ਰਾਜ ਹੋਣ ਕਰਕੇ ਇਸ ਨੂੰ ਅਕਸਰ ਜੰਗਾਂ ਦਾ ਸਾਹਮਣਾ ਕਰਨਾ ਪੈਂਦਾ ਰਿਹਾ ਹੈ ਇੱਥੋਂ ਦੇ ਲੋਕਾਂ ਨੇ ਕਦੇ ਕਿਸੇ ਦੁਸ਼ਮਣ ਦਾ ਡਰ ਨਹੀਂ ਮੰਨਿਆ ਇਹਨਾਂ ਦਾ ਵਿਰਸਾ ਬਹਾਦਰੀ ਭਰਿਆ ਹੈ ਦੇਸ ਦੀ ਅਜ਼ਾਦੀ ਦੀ ਲੜਾਈ ਵਿੱਚ ਵੀ ਪੰਜਾਬੀਆਂ ਨੇ ਵੱਡਾ ਯੋਗਦਾਨ ਪਾਇਆ ਹੈ ਇੱਥੋਂ ਦੇ ਲੋਕ ਆਪਣੀ ਬਹਾਦਰੀ ਕਰਕੇ ਦੁਨੀਆ ਭਰ ਵਿੱਚ ਪ੍ਰਸਿੱਧ ਹਨ

ਪੰਜਾਬ ਦੀ ਬੋਲੀ ਪੰਜਾਬੀ ਹੈ ਪੰਜਾਬੀ ਬੋਲਣ ਵਾਲੇ ਪੰਜਾਬ ਤੋਂ ਬਾਹਰ ਦੂਰ-ਦੂਰ ਤੱਕ ਫੈਲੇ ਹੋਏ ਹਨ ਪੰਜਾਬੀ ਬੋਲਣ ਵਾਲਿਆਂ ਦੀ ਗਿਣਤੀ ਦੇ ਅਧਾਰ ‘ਤੇ ਇਹ ਸੰਸਾਰ ਦੀ ਦਸਵੀਂ-ਗਿਆਰਵੇਂ ਨੰਬਰ ਦੀ ਭਾਸ਼ਾ ਹੈ ਸਾਡੇ ਗੁਰੂਆਂ, ਸੂਫ਼ੀਆਂ, ਫ਼ਕੀਰਾਂ, ਕਵੀਆਂ ਅਤੇ ਕਲਾਕਾਰਾਂ ਨੇ ਇਸ ਭਾਸ਼ਾ ਨੂੰ ਅਪਣਾ ਕੇ ਇਸ ਦਾ ਮਾਣ ਵਧਾਇਆਹੈ ਅੱਜ ਪੰਜਾਬੀ ਨੂੰ ਇੱਕ ਅਮੀਰ ਭਾਸ਼ਾ ਕਰਕੇ ਜਾਣਿਆ ਜਾਂਦਾ ਹੈ।

ਪੰਜਾਬ ਦੇ ਲੋਕ-ਨਾਚ ਭੰਗੜਾ ਅਤੇ ਗਿੱਧਾ ਹਨ ਮਰਦਾਂ ਦੇ ਨਾਚ ਦਾ ਨਾਂ ਭੰਗੜਾ ਹੈ ਅਤੇ ਔਰਤਾਂ ਦੇ ਨਾਚ ਦਾ ਨਾਂ ਗਿੱਧਾਹੈ ਇਹ ਨਾਚ ਕਿਸੇ ਖੁਸ਼ੀ ਦੇ ਮੌਕੇ ਉੱਤੇ ਕੀਤੇ ਜਾਂਦੇ ਹਨ ਨੱਚਣਾ-ਗਾਉਣਾ ਪੰਜਾਬੀਆਂ ਦੀ ਰੂਹ ਦੀ ਖੁਰਾਕ ਹੈ

ਪੰਜਾਬ ਖੇਤੀ-ਬਾੜੀ ਕਰਕੇ ਵੀ ਬਹੁਤ ਪ੍ਰਸਿੱਧ ਹੈ ਇੱਥੋਂ ਦੀ ਧਰਤੀ ਬੜੀ ਉਪਜਾਊ ਹੈ ਇੱਥੇ ਕਣਕ, ਚੌਲ, ਮੱਕੀ ਅਤੇ ਗੰਨੇ ਦੀ ਭਰਪੂਰ ਫ਼ਸਲ ਹੁੰਦੀ ਹੈ ਪੰਜਾਬ ਦਾ ਕੁੱਲ ਖੇਤਰਫਲ ਘੱਟ ਹੋਣ ਦੇ ਬਾਵਜੂਦ ਇਹ ਭਾਰਤ ਦੇ ਅਨਾਜ ਭੰਡਾਰਾਂ ਵਿੱਚ ਵੱਡਾ ਹਿੱਸਾ ਪਾਉਂਦਾ ਹੈ ਖੇਤੀ-ਬਾੜੀ ਦੇ ਨਾਲ-ਨਾਲ ਪਸ਼ੂ-ਪਾਲਣ ਵੀ ਪੰਜਾਬੀਆਂ ਦਾ ਮੁੱਖ ਕਿੱਤਾ ਹੈ

ਪੰਜਾਬੀਆਂ ਨੂੰ ਮੇਲਿਆਂ ਦਾ ਵੀ ਬੜਾ ਚਾਅ ਰਹਿੰਦਾ ਹੈ ਪੰਜਾਬ ਵਿੱਚ ਬਹੁਤ ਸਾਰੇ ਮੇਲੇ ਵੀ ਲਗਦੇ ਹਨ ਇਹਨਾਂ ਮੇਲਿਆਂ ਵਿਚ ਵਿਸਾਖੀ ਦਾ ਮੇਲਾ, ਛਪਾਰ ਦਾ ਮੇਲਾ, ਜਰਗ ਦਾ ਮੇਲਾ, ਹੋਲਾ-ਮਹੱਲਾ, ਮਾਘੀ ਦਾ ਮੇਲਾ, ਪ੍ਰੋ. ਮੋਹਨ ਸਿੰਘ ਮੇਲਾ ਬਹੁਤ ਪ੍ਰਸਿੱਧ ਹਨ

ਪੰਜਾਬੀ ਲੋਕ ਸੁਭਾਅ ਪੱਖੋਂ ਬੜੇ ਖੁੱਲ੍ਹ-ਦਿਲੇ , ਖੁਸ਼-ਮਿਜ਼ਾਜ ਅਤੇ ਦੂਜਿਆਂ ਦੇ ਕੰਮ ਆਉਣ ਵਾਲੇ ਹਨ ਲੋੜਵੰਦਾਂ ਲਈ ਲੰਗਰ ਲਗਾਉਣੇ, ਛਬੀਲਾਂ ਲਗਾਉਣੀਆਂ ਅਤੇ ਦੂਜਿਆਂ ਦੀ ਖੁੱਲ੍ਹੇ ਦਿਲ ਨਾਲ ਮਦਦ ਕਰਨਾ ਇਹਨਾਂ ਦਾ ਮੁੱਢ-ਕਦੀਮੀ ਸੁਭਾਅ ਹੈ।


ਮੌਖਿਕ ਪ੍ਰਸ਼ਨ
1) ਪੰਜਾਬ ਵਿਚ ਪਹਿਲਾਂ ਕਿੰਨੇ ਦਰਿਆ ਸਨ?
ਉੱਤਰ :
ਪੰਜ।
2) ਪੰਜਾਬ ਵਿਚ ਅੱਜ-ਕੱਲ੍ਹ ਕਿਹੜੇ ਤਿੰਨ ਦਰਿਆ ਹਨ?
ਉੱਤਰ :
ਸਤਲੁਜ, ਬਿਆਸ, ਰਾਵੀ।
3) ਪੰਜਾਬ ਵਿਚ ਕਿਹੜੀ ਭਾਸ਼ਾ ਬੋਲੀ ਜਾਂਦੀ ਹੈ?
ਉੱਤਰ :
ਪੰਜਾਬੀ।
4) ਪੰਜਾਬ ਵਿਚ ਕਿਹੜੀਆਂ-ਕਿਹੜੀਆਂ ਫ਼ਸਲਾਂ ਹੁੰਦੀਆਂ ਹਨ?
ਉੱਤਰ :
ਗੰਨਾ, ਕਣਕ, ਚੌਲ, ਮੱਕੀ।
5) ਪੰਜਾਬੀਆਂ ਦੇ ਪ੍ਰਮੁੱਖ ਕਿੱਤੇ ਕਿਹੜੇ-ਕਿਹੜੇ ਹਨ?
ਉੱਤਰ :
ਖੇਤੀਬਾੜੀ।
6) ਪੰਜਾਬ ਦੇ ਮੁੱਖ ਮੇਲੇ ਕਿਹੜੇ-ਕਿਹੜੇ ਹਨ?
ਉੱਤਰ :
ਜਰਗ ਦਾ, ਛਪਾਰ ਦਾ, ਹੌਲਾ ਮਹੱਲਾ, ਵਿਸਾਖੀ ਦਾ, ਪ੍ਰੋ: ਮੋਹਨ ਸਿੰਘ ਮੇਲਾ।

7) ਪੰਜਾਬੀਆਂ ਦਾ ਸੁਭਾਅ ਕਿਹੋ-ਜਿਹਾ ਹੈ?
ਉੱਤਰ :
ਖੁੱਲ੍ਹ ਦਿਲਾ, ਖੁਸ਼ਮਿਜ਼ਾਜ ਅਤੇ ਦੂਸਰਿਆਂ ਦੇ ਕੰਮ ਆਉਣ ਵਾਲੇ।
8) ਤੁਹਾਨੂੰ ਪੰਜਾਬ ਕਿਹੋ-ਜਿਹਾ ਲੱਗਦਾ ਹੈ?
ਉੱਤਰ :
ਬਹੁਤ ਹੀ ਵਧੀਆ।
(ਅ) ਮਾਂ-ਬੋਲੀ ਨਾਲ ਪਿਆਰ

ਅਧਿਆਪਕ : ਬੱਚਿਓ, ਅੱਜ ਅਸੀਂ ਮਾਂ-ਬੋਲੀ ਬਾਰੇ ਗੱਲ ਕਰਾਂਗੇ
ਅੰਕੁਰ : ਸਰ, ਮਾਂ-ਬੋਲੀਕੀ ਹੁੰਦੀ ਹੈ?
ਅਧਿਆਪਕ : ਬੱਚਿਓ, ਮਾਂ-ਬੋਲੀ ਉਹ ਬੋਲੀ ਹੁੰਦੀ ਹੈ, ਜਿਹੜੀ ਬੱਚਾ ਸ਼ੁਰੂ ਤੋਂ ਹੀ ਆਪਣੇ ਮਾਤਾ-ਪਿਤਾ ਜਾਂ ਆਪਣੇ ਪਰਿਵਾਰ ਤੋਂ ਸਿੱਖਦਾ ਹੈ।
ਬਲਜੀਤ : ਸਰ, ਜੇ ਬੱਚਾ ਮਾਤਾ-ਪਿਤਾ ਜਾਂ ਪਰਿਵਾਰ, ਸਾਰਿਆਂ ਤੋਂ ਸਿੱਖਦਾ ਹੈ ਤਾਂ ਫਿਰ ਇਸ ਨੂੰ ਮਾਂ-ਬੋਲੀ ਕਿਉਂ ਕਹਿੰਦੇ ਨੇ?
ਅਧਿਆਪਕ : ਹਾਂ ਬਈ, ਬਲਜੀਤ ਤੂੰ ਬੜਾ ਸੋਹਣਾ ਸਵਾਲ ਪੁੱਛਿਆ ਹੈ ਅਸਲ ਵਿੱਚ ਬੱਚਾ ਸ਼ੁਰੂ ਵਿੱਚ ਸਭ ਤੋਂ ਵੱਧ ਸਮਾਂ ਮਾਂ ਕੋਲ ਹੀ ਰਹਿੰਦਾ ਹੈ ਸ਼ਾਇਦ ਤਾਂ ਹੀ ਇਸ ਦਾ ਨਾਂ ਮਾਂ-ਬੋਲੀ ਪੈ ਗਿਆਹੋਣਾ। ਸਰ, ਹਰਭਜਨ ਮਾਨ ਦਾ ਇਕ ਗੀਤ ਹੈ-ਮੈਨੂੰ ਇਉਂ ਨਾ ਮਨੋਂ ਵਿਸਾਰ, ਵੇ ਮੈਂ ਤੇਰੀ ਮਾਂ ਦੀ ਬੋਲੀ ਹਾਂ
ਅਧਿਆਪਕ : ਸ਼ਾਬਾਸ਼ ! ਪੁੱਤ ਤੂੰ ਬੜੇ ਸੋਹਣੇ ਗੀਤ ਦਾ ਚੇਤਾ ਕਰਵਾਇਆਹੈ
ਬਲਜੀਤ : ਸਰ , ਸਤਿੰਦਰ ਸਰਤਾਜ ਦਾ ਵੀ ਇੱਕ ਗੀਤ ਹੈ- ਮੈਂ ਗੁਰਮੁਖੀ ਦਾ ਬੇਟਾ
ਅਧਿਆਪਕ : ਹਾਂ ਪੁੱਤ, ਉਹ ਵੀ ਬੜਾ ਸੋਹਣਾ ਗੀਤ ਹੈ ਤੁਸੀਂ ਬੜੇ ਸੋਹਣੇ ਗੀਤ ਸੁਣਦੇ ਹੋ ਪੁੱਤ,ਇਹਨਾਂ ਦੋਹਾਂ ਗੀਤਾਂ ਵਿੱਚ ਮਾਂ-ਬੋਲੀ ਦੀ ਮਹੱਤਤਾ ਦੱਸੀ ਹੈ ਮਾਂ-ਬੋਲੀ ਨੂੰ ਪਿਆਰ ਕਰਨ ਦਾ ਸੁਨੇਹਾ ਦਿੱਤਾ ਹੈ।

ਸਵਿਤਾ : ਸਰ ਸਾਡੀ ਮਾਂ-ਬੋਲੀ ਪੰਜਾਬੀ ਹੈ ਨਾ?
ਅਧਿਆਪਕ : ਹਾਂਜੀ, ਸਾਡੀ ਮਾਂ ਬੋਲੀ ਪੰਜਾਬੀ ਹੈ
ਸਵਿਤਾ : ਸਰ, ਫਿਰ ਗੁਰਮੁਖੀ ਕੀ ਹੈ?
ਅਧਿਆਪਕ : ਪੁੱਤ ਗੁਰਮੁਖੀ ਪੰਜਾਬੀ ਭਾਸ਼ਾ ਦੀ ਲਿਪੀ ਹੈ ਪੰਜਾਬੀ ਭਾਸ਼ਾ ਨੂੰ ਲਿਖਣ ਲਈ ਜਿਹੜੇ ਚਿੰਨ੍ਹ ਵਰਤੇ ਜਾਂਦੇ ਨੇ, ਉਹਨਾਂ ਸਾਰਿਆਂ ਚਿੰਨ੍ਹਾਂ ਨੂੰ ਲਿਪੀ ਕਹਿੰਦੇ ਨੇ ਜਿਵੇਂ ਓ ਅ ੲ ਸਿਹਾਰੀ, ਬਿਹਾਰੀ, ਬਿੰਦੀ, ਟਿੱਪੀ ਜਿਹੜੇ ਚਿੰਨ੍ਹ ਵੀ ਲਿਖਣ ਲਈ ਵਰਤੇ ਜਾਂਦੇ ਨੇ, ਉਹਨਾਂ ਦੇ ਸਮੂਹ ਨੂੰ ਲਿਪੀ ਕਿਹਾ ਜਾਂਦਾ ਹੈ ਪੰਜਾਬੀ ਲਿਖਣ ਲਈ ਗੁਰਮੁਖੀ ਲਿਪੀ ਵਰਤੀ ਜਾਂਦੀ ਹੈ
ਸਵਿਤਾ : ਸਰ, ਕਈ ਹਿੰਦੀ ਫ਼ਿਲਮਾਂ ਵਿੱਚ ਪੰਜਾਬੀ ਗੀਤ ਕਿਉਂ ਹੁੰਦੇ ਨੇ?
ਅਧਿਆਪਕ : ਬੱਚਿਓ, ਪੰਜਾਬੀ ਬੜੀ ਪ੍ਰਸਿੱਧ ਭਾਸ਼ਾ ਹੈ ਬੋਲਣ ਵਾਲਿਆਂ ਦੀ ਗਿਣਤੀ ਦੇ ਹਿਸਾਬ ਨਾਲ ਇਹ ਸੰਸਾਰ ਦੀ ਕੋਈ ਦਸਵੇਂ-ਗਿਆਰਵੇਂ ਨੰਬਰ ਦੀ ਭਾਸ਼ਾ ਹੈ ਦੁਨੀਆਂ ਦੇ ਕੋਈ 160 ਤੋਂ ਵੱਧ ਦੇਸ਼ਾਂ ਵਿੱਚ ਪੰਜਾਬੀ ਲੋਕ ਪੁੱਜ ਚੁੱਕੇ ਨੇ ਜਦੋਂ ਕਿਸੇ ਹਿੰਦੀ ਫ਼ਿਲਮ ਵਿੱਚ ਪੰਜਾਬੀ ਗੀਤ ਹੁੰਦਾ ਹੈ ਤਾਂ ਕਈ ਪੰਜਾਬੀ ਬੰਦੇ ਉਸ ਫ਼ਿਲਮ ਨੂੰ ਦੇਖਣਾ ਚਾਹੁੰਦੇ ਨੇ ਹਿੰਦੀ ਫ਼ਿਲਮਾਂਵਾਲੇ ਆਪਣੀਆਂ ਫ਼ਿਲਮਾਂ ਨੂੰ ਹੋਰ ਮਸ਼ਹੂਰ ਕਰਨ ਲਈ ਫ਼ਿਲਮ ਵਿਚ ਕਈ ਵਾਰੀ ਪੰਜਾਬੀ ਗੀਤ ਪਾਲੈਂਦੇ ਨੇ ਭਾਰਤੀ ਸਰ, ਸਾਡੇ ਘਰ ਵਿੱਚ ਸਾਰੇ ਹਿੰਦੀ ਬੋਲਦੇ ਨੇ ਸਾਡਾ ਪੱਕਾ ਘਰ ਯੂ ਪੀ. ਵਿੱਚ ਹੈ ਸਾਡੇ ਰਿਸ਼ਤੇਦਾਰ ਵੀ ਹਿੰਦੀ ਬੋਲਦੇ ਨੇ
ਅਧਿਆਪਕ : ਬੇਟੇ, ਤੁਹਾਡੀ ਮਾਂ-ਬੋਲੀ ਫਿਰ ਹਿੰਦੀ ਹੋਈ ਕੋਈ ਨਾ ਹਿੰਦੀ ਅਤੇ ਪੰਜਾਬੀ ਦੋਵੇਂ ਭੈਣਾਂ ਭੈਣਾਂ ਨੇ ਹਰ ਇੱਕ ਮਨੁੱਖ ਨੂੰ ਆਪਣੀ ਮਾਂ-ਬੋਲੀ ਨਾਲ ਪਿਆਰ ਹੋਣਾ ਚਾਹੀਦਾ ਹੈ ਮਾਂ-ਬੋਲੀ ਦਾ ਸਾਡੇ ਦਿਲ ਅਤੇ ਦਿਮਾਗ ਨਾਲ ਬੜਾ ਡੂੰਘਾ ਅਤੇ ਪਿਆਰਾ ਰਿਸ਼ਤਾ ਹੁੰਦਾ ਹੈ ਮਾਂ-ਬੋਲੀ ਵਿਚ ਸੁਣੀ ਅਤੇ ਪੜੀ ਗੱਲ ਅਸਾਨੀ ਨਾਲ ਸਮਝ ਆ ਜਾਂਦੀ ਹੈ-
ਬਲਜੀਤ: ਹਾਂਜੀ ਸਰ, ਇਹ ਤਾਂ ਹੈ
ਅਧਿਆਪਕ : ਪਰ ਬੱਚਿਓ, ਇਸ ਦਾ ਮਤਲਬ ਇਹ ਵੀ ਨਹੀਂ ਕਿ ਅਸੀਂ ਹੋਰ ਭਾਸ਼ਾਵਾਂ ਨਹੀਂ ਸਿੱਖਣੀਆਂ ਬੱਸ ਇੰਨਾ ਯਾਦ ਰੱਖਣਾ ਹੈ ਕਿ ਮਾਂ-ਬੋਲੀ ਨੂੰ ਭੁਲਾ ਕੇ ਹੋਰ ਭਾਸ਼ਾਵਾਂ ਨਹੀਂ ਸਿੱਖਣੀਆਂ ਹਰਭਜਨ ਮਾਨ ਇਹੋ ਕਹਿ ਰਿਹਾ ਹੈ-ਮੈਨੂੰ ਇਉਂ ਨਾ ਮਨੋ ਵਿਸਾਰ
ਬਲਜੀਤ : ਸਰ, ਮੇਰੇ ਪਾਪਾ ਦੇ ਫ਼ੋਨ ਵਿੱਚ ਮਨਮੋਹਨ ਵਾਰਿਸ ਦਾ ਇਕ ਗੀਤ ਹੈ, ਮਾਂਵਾਂ ਤਿੰਨ ਹੁੰਦੀਆਂ

ਅਧਿਆਪਕ : ਬਲਜੀਤ ਤੇਰੇ ਪਾਪਾ ਵੀ ਬੜੇ ਸੋਹਣੇ ਗੀਤ ਸੁਣਦੇ ਨੇ ਮੈਂ ਵੀ ਉਹ ਗੀਤ ਸੁਣਿਆ ਹੈ ਕਿ ਮਾਂਵਾਂ ਤਿੰਨ ਹੁੰਦੀਆਂ ਨੇ, ਇਕ ਮਾਂ ਧਰਤੀ, ਦੂਜੀ ਮਾਂ ਮਾਂ-ਬੋਲੀ, ਤੀਜੀ ਮਾਂ ਜਨਮਦਾਤੀ ਸੱਚੀ ਬੱਚਿਓ, ਇਹ ਤਿੰਨੋਂ ਸਾਨੂੰ ਪਾਲਦੀਆਂ ਨੇ ਇਹਨਾਂ ਦਾ ਕਰਜ਼ਾ ਨਹੀਂ ਲਾਹਿਆ ਜਾ ਸਕਦਾ। ਸਵਿਤਾ: ਸਰ, ਮੇਰੇ ਚਾਚਾ ਜੀ ਪੰਜਾਬੀ ਦੀਆਂ ਕਿਤਾਬਾਂ ਪੜਦੇ ਨੇ
ਅਧਿਆਪਕ : ਹਾਂ ਬੱਚਿਓ, ਪੰਜਾਬੀ ਵਿੱਚ ਗੁਰਦਿਆਲ ਸਿੰਘ, ਸ਼ਿਵ ਕੁਮਾਰ, ਸੁਰਜੀਤ ਪਾਤਰ ਅਤੇ ਨਰਿੰਦਰ ਸਿੰਘ ਕਪੂਰ ਵਰਗੇ ਲੇਖਕਾਂ ਦੀਆਂ ਕਿਤਾਬਾਂ ਬਹੁਤ ਪੜ੍ਹੀਆਂ ਜਾਂਦੀਆਂ ਨੇ
ਅੰਕੁਰ : ਸਰ, ਮੇਰੇ ਵੀਰ ਜੀ ਕੰਪਿਊਟਰ ਵਿੱਚ ਪੰਜਾਬੀ ਅਖ਼ਬਾਰ ਪੜ੍ਹਦੇ ਨੇ
ਅਧਿਆਪਕ : ਹਾਂ ਬੱਚਿਓ, ਕੰਪਿਊਟਰ ‘ਤੇ ਵੀ ਪੰਜਾਬੀ ਦਾ ਬੜਾ ਕੁਝ ਹੈਗਾ ਮੈਂ ਵੀ ਸਵੇਰੇ ਜਲਦੀ ਉੱਠ ਕੇ ਇੰਟਰਨੈੱਟ ‘ਤੇ ਹੀ ਤਿੰਨ ਪੰਜਾਬੀ ਅਖ਼ਬਾਰਾਂ ਪੜ੍ਹਦਾ ਹਾਂ-ਪੰਜਾਬੀ ਟ੍ਰਿਬਿਊਨ, ਨਵਾਂ ਜ਼ਮਾਨਾ ਅਤੇ
ਅਜੀਤ : ਚਲੋ ਬੱਚਿਓ !ਆਪਣੀ ਗੱਲ-ਬਾਤ ਏਥੇ ਹੀ ਬੰਦ ਕਰਦੇ ਹਾਂ ਫਿਰ ਅੱਜ ਕੀ ਸਿੱਖਿਆ ਤੁਸੀਂ ? ਭਾਰਤੀ ਸਰ, ਅੱਜ ਇਹ ਸਿੱਖਿਆ ਕਿ ਸਾਨੂੰ ਆਪਣੀ ਮਾਂ-ਬੋਲੀ ਕਦੇ ਨਹੀਂ ਭੁਲਾਉਣੀ ਚਾਹੀਦੀ
ਅਧਿਆਪਕ : ਬਿਲਕੁਲ ਠੀਕ ! ਚਲੋ, ਲਹਿੰਦੇ ਪੰਜਾਬ ਦੇ ਇਕ ਪੰਜਾਬੀ ਕਵੀ ਦੀਆਂ ਲਾਈਨਾਂ ਨਾਲ ਇਹ ਗੱਲ ਖ਼ਤਮ ਕਰਦੇ ਹਾਂ –

ਮੈਨੂੰ ਕਈਆਂਨੇ ਆਖਿਆਕਈਵਾਰੀ,
ਤੂੰ ਲੈਣਾ ਪੰਜਾਬੀ ਦਾਨਾਂ ਛੱਡ ਦੇ
ਜਿਦੀ ਗੋਦੀ ਚ ਪਲ ਕੇ ਜਵਾਨ ਹੋਇਓ,
ਜਾਈਆਂ ਉਹ ਮਾਂ ਛੱਡ ਦੇ ਤੇ ਗਰਾਂ ਛੱਡ ਦੇ
ਦਿਓ ਜੇ ਪੰਜਾਬੀ-ਪੰਜਾਬੀਈ ਕੂਕਣਾਈ,
ਖਲੋਤਾਉਹ ਥਾਂ ਛੱਡ ਦੇ ਪੰਜਾਬੀ ਨਾ
ਭੁਲਾ ਦਿਓ। ਮੈਨੂੰ ਇੰਝ ਲੱਗਦਾਲੋਕੀਂ
ਆਖਦੇ ਨੇ, ਤੂੰ ਪੁੱਤਰਾ ਆਪਣੀ ਮਾਂ ਛੱਡ ਦੇ

ਮੌਖਿਕ ਪ੍ਰਸ਼ਨ :
1) ਮਨੁੱਖ ਦੀਆਂ ਕਿਹੜੀਆਂ ਤਿੰਨ ਮਾਂਵਾਂ ਹੁੰਦੀਆਂ ਹਨ?
ਉੱਤਰ :
ਧਰਤੀ ਮਾਂ, ਮਾਂ-ਬੋਲੀ, ਜਨਮ-ਦਾਤੀ ਮਾਂ।
2) ਪੰਜਾਬੀਆਂ ਦੀ ਮਾਂ-ਬੋਲੀ ਕਿਹੜੀ ਹੈ?
ਉੱਤਰ :
ਪੰਜਾਬੀ।
3) ਕੀ ਕੰਪਿਊਟਰ ‘ਤੇ ਵੀ ਪੰਜਾਬੀ ਲਿਖੀ ਜਾਂ ਪੜ੍ਹੀ ਜਾ ਸਕਦੀ ਹੈ?
ਉੱਤਰ :
ਹਾਂ ਜੀ।
4) ਅੱਜ ਦੀ ਗੱਲ-ਬਾਤ ਵਿੱਚੋਂ ਅਸੀਂ ਕੀ ਸਿੱਖਿਆ ਹੈ?
ਉੱਤਰ :
ਸਾਨੂੰ ਆਪਣੀ ਮਾਂ ਬੋਲੀ ਪੰਜਾਬੀ ਨੂੰ ਮਨੋਂ ਵਿਸਾਰ ਕੇ ਹੋਰ ਭਾਸ਼ਾਵਾਂ ਨਹੀਂ ਸਿਖਣੀਆਂ ਚਾਹੀਦੀਆਂ। ਪਰ ਦੂਸਰੀਆਂ ਭਾਸ਼ਾਵਾਂ ਵੀ ਸਿੱਖਣੀਆਂ ਜ਼ਰੂਰ ਚਾਹੀਦੀਆਂ। ਹਨ। ਗਾਇਕਾਂ ਅਤੇ ਲੇਖਕਾਂ ਦੇ ਨਾਂ ਪਤਾ ਲੱਗੇ।
(ੲ) ਦੇਸ ਦੀਆਂ ਹੋਰ ਬੋਲੀਆਂ ਬਾਰੇ
ਜਿਵੇਂ ਸਾਡੀ ਮਾਂ-ਬੋਲੀ ਪੰਜਾਬੀ ਹੈ, ਉਵੇਂ ਹੀ ਹੋਰ ਲੋਕਾਂ ਦੀਆਂ ਮਾਂ-ਬੋਲੀਆਂ ਹਨ ਉਹ ਵੀ ਸਾਡੇ ਵਾਂਗ ਆਪਣੀ ਮਾਂ-ਬੋਲੀ ਨੂੰ ਪਿਆਰ ਕਰਦੇ ਨੇ ਕਈ ਤਾਂ ਸਗੋਂ ਸਾਡੇ ਨਾਲੋਂ ਵੀ ਜ਼ਿਆਦਾ ਆਪਣੀ ਮਾਂ-ਬੋਲੀ ਨੂੰ ਪਿਆਰ ਕਰਦੇ ਨੇ ਹਰ ਇੱਕ ਨੂੰ ਆਪਣੀ ਮਾਂ-ਬੋਲੀ ਨੂੰ ਪਿਆਰ ਕਰਨ ਦਾ ਹੱਕ ਹੈ ਹਰ ਇੱਕ ਨੂੰ ਆਪਣੀ ਮਾਂ-ਬੋਲੀ ਨੂੰ ਪਿਆਰ ਕਰਨਾ ਹੀ ਚਾਹੀਦਾ ਹੈ ਭਾਰਤ ਵਿੱਚ ਸੈਂਕੜੇ ਭਾਸ਼ਾਵਾਂ ਅਤੇ ਉਪ-ਭਾਸ਼ਾਵਾਂ ਬੋਲੀਆਂ ਜਾਂਦੀਆਂ ਹਨ ਇਹਨਾਂ ਵਿੱਚੋਂ ਜ਼ਿਆਦਾ ਬੋਲੀਆਂ ਜਾਣ ਵਾਲੀਆਂ ਕੁਝ ਭਾਸ਼ਾਵਾਂ ਨੂੰ ਸੰਵਿਧਾਨ ਵਿੱਚ ਖ਼ਾਸ ਦਰਜਾ ਦਿੱਤਾ ਗਿਆ ਹੈ
ਤੁਸੀਂ ਕਿਸੇ ਭਾਰਤੀ ਰੁਪਏ ਦਾ ਨੋਟ ਦੇਖਿਆ ਹੋਵੇਗਾ ਇਸ ਉੱਤੇ ਪੰਜਾਬੀ, ਹਿੰਦੀ ਅਤੇ ਅੰਗਰੇਜ਼ੀ ਸਮੇਤ 17 ਭਾਸ਼ਾਵਾਂ ਵਿੱਚ ਨੋਟ ਰਾਸ਼ੀ ਲਿਖੀ ਮਿਲਦੀ ਹੈ ਅਸਲ ਵਿੱਚ ਭਾਰਤ ਇੱਕ ਬਹੁ-ਭਾਸ਼ੀ ਦੇਸ ਹੈ। ਭਾਰਤ ਵੱਖ-ਵੱਖ ਭਾਸ਼ਾਵਾਂ ਦੇ ਫੁੱਲਾਂ ਦਾ ਗੁਲਦਸਤਾ ਹੈ ਇਸ ਕਰ ਕੇ ਭਾਰਤ ਸਰਕਾਰ ਹਰ ਭਾਸ਼ਾ ਨੂੰ ਉਸ ਦਾ ਬਣਦਾ ਹੱਕ ਦਿੰਦੀ ਹੈ ਸਾਨੂੰ ਆਪਣੀ ਭਾਸ਼ਾ ਨੂੰ ਪਿਆਰ ਕਰਨਾ ਚਾਹੀਦਾ ਹੈ ਪਰ ਸਾਨੂੰ ਕਿਸੇ ਹੋਰ ਦੀ ਭਾਸ਼ਾ ਨੂੰ ਨਿੰਦਣਾਵੀਨਹੀਂ ਚਾਹੀਦਾ ਕਿਸੇ ਹੋਰ ਨੂੰ ਵੀ ਆਪਣੀ ਭਾਸ਼ਾਓਨੀ ਹੀ ਪਿਆਰੀ ਹੁੰਦੀ ਹੈ, ਜਿੰਨੀ ਸਾਨੂੰ ਆਪਣੀ ਭਾਸ਼ਾਹੁੰਦੀ ਹੈ


ਸਾਨੂੰ ਆਪਣੀ ਮਾਂ-ਬੋਲੀ ਤਾਂ ਚੰਗੀ ਤਰ੍ਹਾਂ ਸਿੱਖਣੀ ਚਾਹੀਦੀ ਹੈ, ਬੋਲਣੀ ਵੀ ਚਾਹੀਦੀ ਹੈ, ਪਰ ਹੋਰ ਬੋਲੀਆਂ ਵੀ ਸਿੱਖਣੀਆਂ ਚਾਹੀਦੀਆਂ ਹਨ ਹਿੰਦੀ ਅਤੇ ਅੰਗਰੇਜ਼ੀ ਸਿੱਖੇ ਬਿਨਾਂ ਤਾਂ ਬਿਲਕੁਲ ਗੁਜ਼ਾਰਾ ਨਹੀਂ ਜੇ ਸਾਨੂੰ ਭਾਰਤ ਦੇ ਕਿਸੇ ਹੋਰ ਰਾਜਵਿੱਚ ਲੰਮੇ ਸਮੇਂ ਤੱਕ ਰਹਿਣਾ ਪੈ ਜਾਵੇ ਤਾਂ ਸਾਨੂੰ ਉੱਥੋਂ ਦੀ ਭਾਸ਼ਾ ਵੀ ਸਿੱਖ ਲੈਣੀ ਚਾਹੀਦੀ ਹੈ ਇਹ ਸਿੱਖ ਲੈਣ ਨਾਲ ਸਾਡੀਆਂ ਕਈ ਮੁਸ਼ਕਲਾਂ ਹੱਲ ਹੋ ਜਾਣਗੀਆਂ ਵੈਸੇ ਵੀ ਅਸੀਂ ਜਿੰਨੀਆਂ ਵੱਧ ਭਾਸ਼ਾਵਾਂ ਸਿੱਖ ਲੈਂਦੇ ਹਾਂ, ਸਾਡੇ ਲਈ ਗਿਆਨ ਦੇ ਓਨੇ ਦਰਵਾਜ਼ੇ ਖੁੱਲ੍ਹ ਜਾਂਦੇ ਹਨ ਭਾਰਤ ਵਿੱਚ ਹੋਰ ਬੋਲੀਆਂ ਜਾਣ ਵਾਲੀਆਂ ਭਾਸ਼ਾਵਾਂ ਬੰਗਲਾ, ਅਸਾਮੀ, ਮਰਾਠੀ, ਕਸ਼ਮੀਰੀ, ਬੋਡੋ, ਕੰਨੜ, ਮਲਿਆਲਮ, ਗੁਜਰਾਤੀ, ਡੋਗਰੀ, ਉਰਦੂ ਅਤੇ ਤਾਮਿਲ ਆਦਿ ਹਨ

ਕੀ ਤੁਹਾਨੂੰ ਪਤਾ ਹੈ ਕਿ ਮੁਨਸ਼ੀ ਪ੍ਰੇਮ ਚੰਦ ਹਿੰਦੀ ਦੇ ਬਹੁਤ ਵੱਡੇ ਲੇਖਕ ਸਨ ਰਾਬਿੰਦਰ ਨਾਥ ਟੈਗੋਰ ਦਾ ਨਾਂ ਤਾਂ ਤੁਸੀਂ ਸੁਣਿਆ ਹੀ ਹੋਵੇਗਾ ਟੈਗੋਰ ਬੰਗਾਲੀ ਸਨ ਉਹ ਬੰਗਲਾ ਭਾਸ਼ਾ ਵਿੱਚ ਲਿਖਦੇ ਸਨ ਭਾਰਤ ਦਾ ਰਾਸ਼ਟਰੀ ਗਾਣ ‘ਜਨ-ਗਣ-ਮਨ ਉਹਨਾਂ ਦਾ ਹੀ ਲਿਖਿਆ ਹੋਇਆ ਹੈ ਸੋ ਹੋਰ ਭਾਸ਼ਾਵਾਂ ਦੀ ਮਹੱਤਤਾ ਸਮਝਣੀ ਵੀ ਬਹੁਤ ਜ਼ਰੂਰੀ ਹੈ

ਦੇਸ ਮੇਰੇ ਦੀਆਂ ਸਭ ਬੋਲੀਆਂ ਨਿਆਰੀਆਂ
ਦੇਸ ਦੀ ਅਮੀਰੀ ਇਹ ਦਿਖਾਉਣ ਸਾਰੀਆਂ
ਜਿੰਨੀਆਂ ਭਾਸ਼ਾਵਾਂ ਅਸੀਂ ਸਿੱਖ ਲੈਂਦੇ ਹਾਂ,
ਗਿਆਨ ਦੀਆਂਓਨੀਆਂ ਖੁੱਲ੍ਹਣ ਬਾਰੀਆਂ
ਮੌਖਿਕ ਪ੍ਰਸ਼ਨ
1) ਭਾਰਤ ਵਿਚ ਸੈਂਕੜੇ ਭਾਸ਼ਾਵਾਂ ਬੋਲੀਆਂ ਜਾਂਦੀਆਂ ਹਨ ਠੀਕ ਹੈ ਕਿ ਗਲਤ?
ਉੱਤਰ :
ਠੀਕ।
2) ਪੰਜਾਬੀ, ਹਿੰਦੀ ਅਤੇ ਅੰਗਰੇਜ਼ੀ ਤੋਂ ਇਲਾਵਾ ਕੋਈ ਦੋ ਹੋਰ ਭਾਸ਼ਾਵਾਂ ਦੇ ਨਾਂਦੱਸੇ
ਉੱਤਰ :
ਮਰਾਠੀ, ਬੋਡੋ, ਕਸ਼ਮੀਰੀ।
3) ਰਾਬਿੰਦਰ ਨਾਥ ਟੈਗੋਰ ਕਿੱਥੋਂ ਦੇ ਰਹਿਣ ਵਾਲੇ ਸਨ?
ਉੱਤਰ :
ਬੰਗਾਲ ਦੇ।
4) ਵੱਧ ਭਾਸ਼ਾਵਾਂ ਸਿੱਖਣ ਦਾ ਕੋਈ ਲਾਭ ਹੁੰਦਾ ਹੈ ਕਿ ਨਹੀਂ?
ਉੱਤਰ :
ਸਾਡੇ ਲਈ ਗਿਆਨ ਦੇ ਹੋਰ ਦਰਵਾਜ਼ੇ ਖੁੱਲ੍ਹ ਜਾਂਦੇ ਹਨ।

(ਸ) ਦੇਸ ਦੇ ਸਾਰੇ ਲੋਕਾਂ ਨਾਲ ਪਿਆਰ
ਮਨੁੱਖ ਦੇ ਦਿਲ ਵਿੱਚ ਸੱਚਾ ਦੇਸ-ਪਿਆਰ ਹੋਣਾ ਇੱਕ ਬਹੁਤ ਵੱਡਾ ਮਨੁੱਖੀ ਗੁਣ ਹੈ ਇਹ ਪਿਆਰ ਨਿਰਾ ਦੇਸ ਦੀਆਂ ਚੀਜ਼ਾਂ-ਵਸਤਾਂ ਨਾਲ ਪਿਆਰ ਨਹੀਂ ਹੁੰਦਾ ਅਸਲੀ ਦੇਸ-ਪਿਆਰ ਦੇਸ ਦੇ ਲੋਕਾਂ ਨਾਲ ਪਿਆਰ ਹੁੰਦਾ ਹੈ ਦੇਸ ਦੇ ਲੋਕਾਂ ਤੋਂ ਭਾਵ ਦੇਸ ਦੇ ਸਾਰੇ ਲੋਕਾਂ ਤੋਂ ਹੈ ਜਦੋਂ ਤੱਕ ਅਸੀਂ ਦੇਸ ਦੇ ਸਾਰੇ ਲੋਕਾਂ ਨੂੰ ਪਿਆਰ ਨਹੀਂ ਕਰਦੇ, ਅਸੀਂ ਉਦੋਂ ਤੱਕ ਇਹ ਨਹੀਂ ਕਹਿ ਸਕਦੇ ਕਿ ਅਸੀਂ ਦੇਸ ਨੂੰ ਪਿਆਰ ਕਰਦੇ ਹਾਂ ਜਿਵੇਂ ਕਿ ਤੁਸੀਂ ਜਾਣਦੇ ਹੋ ਕਿ ਅਸੀਂ ਸਾਰੇ ਮਨੁੱਖ ਹਾਂ ਇਸ ਲਈ ਸਾਡੇ ਮਨ ਵਿੱਚ ਕਿਸੇ ਦੂਜੇ ਮਨੁੱਖ ਪ੍ਰਤੀ ਕੋਈ ਵੈਰ-ਵਿਰੋਧ ਜਾਂ ਵਿਤਕਰਾ ਨਹੀਂ ਹੋਣਾ ਚਾਹੀਦਾ
ਕੋਈ ਨਾਗਰਿਕ ਭਾਵੇਂ ਉਹ ਕਿਸੇ ਵੀ ਰਾਜ ਵਿੱਚ ਰਹਿੰਦਾ ਹੈ, ਕਿਸੇ ਵੀ ਧਰਮ ਨੂੰ ਮੰਨਦਾ ਹੈ, ਕਿਸੇ ਵੀ ਜਾਤੀ ਨਾਲ ਸੰਬੰਧ ਰੱਖਦਾ ਹੈ ਅਤੇ ਕੋਈ ਵੀ ਭਾਸ਼ਾ ਬੋਲਦਾ ਹੈ, ਉਹ ਦੇਸ ਦਾ ਹਿੱਸਾ ਹੈ ਉਸ ਨਾਲ ਕਿਸੇ ਤਰ੍ਹਾਂ ਦਾ ਵਿਤਕਰਾ ਨਹੀਂ ਹੋਣਾ ਚਾਹੀਦਾ ਸਾਡੇ ਵਿੱਚ ਕੋਈ ਉਚ-ਨੀਚ ਦੀ ਭਾਵਨਾ ਨਹੀਂ ਹੋਣੀ ਚਾਹੀਦੀ ਹਰ ਨਾਗਰਿਕ ਨੂੰ ਦੂਜੇ ਦਾ ਮਦਦਗਾਰ ਹੋਣਾ ਚਾਹੀਦਾ ਹੈ ਕੋਈ ਮਨੁੱਖ ਕਿਸੇ ਦੂਜੇ ਮਨੁੱਖ ਦਾ ਕੋਈ ਨੁਕਸਾਨ ਕਰਨ ਬਾਰੇ ਨਾ ਸੋਚੇ ਕੋਈ ਕਿਸੇ ਨਾਲ ਕੋਈ ਹੇਰਾ-ਫੇਰੀ ਨਾ ਕਰੇ ਇਸ ਤਰ੍ਹਾਂ ਕਰਨਾ ਹੀ ਦੇਸ ਦੇ ਲੋਕਾਂ ਨੂੰ ਪਿਆਰ ਕਰਨਾ ਹੁੰਦਾ ਹੈ
ਸਭ ਤੋਂ ਮਹੱਤਵਪੂਰਨ ਗੱਲ ਹੈ, ਦੇਸ ਦੇ ਗਰੀਬ ਲੋਕਾਂ ਦੇ ਹੱਕਾਂ ਬਾਰੇ ਸੋਚਣਾ ਸਾਨੂੰ ਗ਼ਰੀਬ ਲੋਕਾਂ ਦਾ ਜੀਵਨ ਸੁਖਾਲਾ ਬਣਾਉਣ ਦੀ ਕੋਸ਼ਿਸ਼ ਕਰਨੀ ਚਾਹੀਦੀ ਹੈ ਉਹਨਾਂ ਨੂੰ ਪੜ੍ਹਨ-ਲਿਖਣ ਵਿੱਚ ਮਦਦ ਕਰਨੀ ਚਾਹੀਦੀ ਹੈ ਦੇਸ ਤਾਂ ਹੀ ਖ਼ੁਸ਼ਹਾਲ ਹੋ ਸਕਦਾ ਹੈ, ਜੇ ਦੇਸ ਦੇ ਸਾਰੇ ਲੋਕ ਖੁਸ਼ ਹੋਣ, ਉਹਨਾਂ ਕੋਲ ਜੀਵਨ ਦੀਆਂ ਜ਼ਰੂਰੀ ਸਹੂਲਤਾਂ ਹੋਣ ਕਿਸੇ ਕਮਜ਼ੋਰ ਨੂੰ ਨਾਲ ਲੈ ਕੇ ਚੱਲਣਾ ਹੀਤਾਂ ਅਸਲੀ ਨੇਕੀ ਹੈ ਆਓ, ਇਕ ਕਵਿਤਾ ਪੜਦੇ ਹਾਂ:

ਆਓ, ਸਾਰੇ ਲੋਕਾਂ ਨੂੰ ਪਿਆਰ ਕਰੀਏ,
ਦਿਲਾਂ ਚ ਕਦੇ ਨਾਨਫ਼ਰਤ ਭਰੀਏ।
ਸਾਰੇ ਹੀ ਨੇ ਆਪਣੇ, ਬੇਗਾਨਾ ਕੋਈ ਨਾ,
ਪਿਆਰ ਜਿਹਾ ਜੱਗ ਚਤਰਾਨਾ ਕੋਈ ਨਾ।
ਸੱਚੇ-ਸੁੱਚੇ ਦਿਲ ਚ ਵਿਚਾਰ ਰੱਖੀਏ,
ਦਿਲ ’ਚ ਨਾਕਦੇ ਹੰਕਾਰ ਰੱਖੀਏ।
ਵੰਡੀਆਂਦਿਲਾਂ ਦੇ ਵਿੱਚ ਆਉਣ ਦੇਈਏ ਨਾ,
ਝਗੜਾ ਕਿਸੇ ਨੂੰ ਕਦੇ ਪਾਉਣ ਦੇਈਏ ਨਾ।
ਮੌਖਿਕ ਪ੍ਰਸ਼ਨ:
1) ਕੀ ਅਸੀਂ ਸਾਰੇ ਭਾਰਤੀ ਇੱਕ ਹਾਂ?
ਉੱਤਰ :
ਹਾਂ ਅਸੀਂ ਸਾਰੇ ਭਾਰਤੀ ਇੱਕ ਹਾਂ।
2) ਸਾਨੂੰ ਦੂਜਿਆਂ ਦੀ ਮਦਦ ਕਰਨੀ ਚਾਹੀਦੀ ਹੈ ਕਿ ਨੁਕਸਾਨ?
ਉੱਤਰ :
ਮਦਦ ਕਰਨੀ ਚਾਹੀਦੀ ਹੈ।
3) ਪਿਆਰ ਨਾਲ ਰਹਿਣ ਦੇ ਕੀਲਾਭ ਹਨ?
ਉੱਤਰ :
ਸਾਰਿਆਂ ਦੀ ਅਤੇ ਦੇਸ਼ ਦੀ ਤਰੱਕੀ ਹੁੰਦੀ ਹੈ।

4) ਦੇਸ ਵੀ ਤਾਂ ਇਕ ਵੱਡਾ ਪਰਿਵਾਰ ਹੈ ਠੀਕ ਹੈ ਕਿ ਗ਼ਲਤ ?
ਉੱਤਰ :
ਠੀਕ।
5) ਕੀ ਸਾਰੇ ਮਨੁੱਖਾਂ ਦਾ ਖੂਨ ਇੱਕੋ-ਜਿਹਾ ਹੁੰਦਾ ਹੈ ਕਿ ਵੱਖੋ-ਵੱਖਰਾ?
ਉੱਤਰ :
ਇਕੋ ਜਿਹਾ।
6) ਕੀ ਧਰਮ,ਜਾਤ ਜਾਂ ਇਲਾਕੇ ਦੇ ਅਧਾਰ ‘ਤੇ ਲੜਨਾ ਚੰਗੀ ਗੱਲ ਹੈ?
ਉੱਤਰ :
ਨਹੀਂ, ਇਹ ਬਹੁਤ ਮਾੜੀ ਗੱਲ ਹੈ।
7) ਕੀਤੁਸੀਂ ਵੱਡੇ ਹੋ ਕੇ ਲੋਕਾਂ ਨੂੰ ਪਿਆਰ ਨਾਲ ਰਹਿਣ ਲਈ ਸਮਝਾਉਗੇ?
ਉੱਤਰ :
ਹਾਂ, ਮੈਂ ਹੁਣ ਤੋਂ ਹੀ ਸ਼ੁਰੂ ਕਰ ਦਿਆਂਗਾ।
PSEB 5th Class Welcome Life Guide ਦਿਲ ਵਿੱਚ ਇਹਨਾਂ ਲਈ ਪਿਆਰ ਰੱਖੀਏ Important Questions and Answers
ਹੋਰ ਮਹੱਤਵਪੂਰਨ ਪ੍ਰਸ਼ਨ
ਬਹੁਵਿਕਲਪੀ ਪ੍ਰਸ਼ਨ :
1. ਹੁਣ ਦੇ ਪੰਜਾਬ ਵਿਚ ਕਿਹੜਾ ਦਰਿਆ ਨਹੀਂ ਹੈ ?
(ਉ) ਸਤਲੁਜ
(ਅ) ਜਿਹਲਮ
(ਇ) ਬਿਆਸ
(ਸ) ਰਾਵੀ।
ਉੱਤਰ :
(ਅ) ਜਿਹਲਮ।

2. ਦੇਸ਼ ਕਦੋਂ ਅਜ਼ਾਦ ਹੋਇਆ ?
(ਉ) 1950
(ਅ) 1947
(ਇ) 1974
(ਸ) 1945.
ਉੱਤਰ :
(ਅ) 1947.
3. ਪੰਜਾਬ ਕਿਹੋ ਜਿਹਾ ਰਾਜ ਹੈ ?
(ਉ) ਸਰਹੱਦੀ
(ਅ) ਸਮੁੰਦਰ ਕਿਨਾਰੇ
(ਈ) ਰੇਤਲਾ
(ਸ) ਸਾਰੇ ਗਲਤ।
ਉੱਤਰ :
(ੳ) ਸਰਹੱਦੀ
4. ਭਾਰਤੀ ਰੁਪਏ ਤੇ ਕਿੰਨੀਆਂ ਭਾਸ਼ਾਵਾਂ ਹੁੰਦੀਆਂ ਹਨ ?
(ਉ) 10
(ਅ) 17
(ਈ) 11
(ਸ) 21.
ਉੱਤਰ :
(ਅ) 17.9
5. ਟੈਗੋਰ ਕਿਸ ਭਾਸ਼ਾ ਵਿਚ ਲਿਖਦੇ ਸਨ ?
(ਉ) ਪੰਜਾਬੀ
(ਅ) ਬੰਗਾਲੀ
(ੲ) ਉਰਦੂ
(ਸ) ਹਿੰਦੀ !
ਉੱਤਰ :
(ਅ) ਬੰਗਾਲੀ।

6. ਮੁਨਸ਼ੀ ਪ੍ਰੇਮ ਚੰਦ ਕੀ ਸਨ ?
(ਉ) ਲਿਖਾਰੀ
(ਅ) ਕਵੀ
(ਬ) ਐਕਟਰ
(ਸ) ਗੀਤਕਾਰ।
ਉੱਤਰ :
(ੳ) ਲਿਖਾਰੀ।
7. ਭਾਰਤ ਦਾ ਰਾਸ਼ਟਰ ਗਾਣ ਕਿਸ ਨੇ ਲਿਖਿਆ ਹੈ ?
(ਉ) ਟੈਗੋਰ
(ਅ) ਮੁਨਸ਼ੀ ਪ੍ਰੇਮ ਚੰਦ
(ੲ) ਅੰਮ੍ਰਿਤਾ ਪ੍ਰੀਤਮ
(ਸ) ਕੋਈ ਨਹੀਂ।
ਉੱਤਰ :
(ੳ) ਟੈਗੋਰ।
8. ਕਿੰਨੇ ਦੇਸ਼ਾਂ ਵਿਚ ਪੰਜਾਬੀ ਲੋਕ ਪੁੱਜ ਚੁੱਕੇ ਹਨ ?
(ਉ) 160 ਤੋਂ ਵੱਧ
(ਅ) 95 ਤੋਂ ਘੱਟ
(ਇ) 340
(ਸ) 460.
ਉੱਤਰ :
(ੳ) 160 ਤੋਂ ਵੱਧ।
9. ਕਿਸਨੇ ਗੀਤ ਵਿਚ ਕਿਹਾ ਹੈ ਕਿ ਮਾਂਵਾਂ ਤਿੰਨ ਹੁੰਦੀਆਂ ਹਨ ?
(ਉ) ਸਰਤਾਜ
(ਅ) ਬਟਾਲਵੀ
(ਈ) ਮਨਮੋਹਨ ਵਾਰਿਸ
(ਸ) ਹਰਭਜਨ ਮਾਨ।
ਉੱਤਰ :
(ਈ) ਮਨਮੋਹਨ ਵਾਰਿਸ।

10. ਮਸ਼ਹੂਰ ਲੇਖਕ ਹਨ ?
(ਉ) ਸ਼ਿਵ ਕੁਮਾਰ ਬਟਾਲਵੀ
(ਅ) ਨਰਿੰਦਰ ਸਿੰਘ ਕਪੂਰ
(ਈ) ਗੁਰਦਿਆਲ ਸਿੰਘ
(ਸ) ਸਾਰੇ ਠੀਕ।
ਉੱਤਰ :
(ਸ) ਸਾਰੇ ਠੀਕ।
11. ਸਾਨੂੰ ਆਪਣੀ ਮਾਂ-ਬੋਲੀ ਨਾਲ … ਕਰਨਾ ਚਾਹੀਦਾ ਹੈ।
(ਉ) ਪਿਆਰ
(ਅ) ਵਿਤਕਰਾ
(ਈ) ਨਫ਼ਰਤ
(ਸ) ਉਪਰੋਕਤ ਸਭ ਕੁਝ।
ਉੱਤਰ :
(ੳ) ਪਿਆਰ।
12. ਅਧਿਆਪਕ ਜੀ ਕੰਪਿਊਟਰ ‘ਤੇ ਕਿਹੜੀ ਅਖਵਾਰ ਪੜ੍ਹਦੇ ਹਨ ?
(ਉ) ਪੰਜਾਬੀ ਟ੍ਰਿਬਿਊਨ
(ਅ) ਨਵਾਂ ਜ਼ਮਾਨਾ
(ਬ) ਅਜੀਤ
(ਸ) ਸਾਰੇ ਠੀਕ।
ਉੱਤਰ :
(ਸ) ਸਾਰੇ ਠੀਕ।
ਖਾਲੀ ਥਾਂਵਾਂ ਭਰੋ :
1. ਅਸੀਂ …………………………… ਰਾਜ ਦੇ ਵਾਸੀ ਹਾਂ।
2. ਪੰਜਾਬ ਦੇ ਲੋਕ ਆਪਣੀ …………………………… ਕਾਰਨ ਦੁਨੀਆ ਭਰ ਵਿਚ ਪ੍ਰਸਿੱਧ ਹਨ।
3. ਪੰਜਾਬੀ ਬੋਲਣ ਵਾਲਿਆਂ ਦੀ ਗਿਣਤੀ …………………………… ਦੇ ਆਧਾਰ ‘ਤੇ ਇਹ ਸੰਸਾਰ ਵਿੱਚ।
4. …………………………… ਨੰਬਰ ਦੀ ਭਾਸ਼ਾ ਹੈ। ਰਾਜ ਹੋਣ ਕਾਰਨ ਪੰਜਾਬ ਨੂੰ ਅਕਸਰ ਜੰਗਾਂ ਦਾ ਸਾਹਮਣਾ ਕਰਨਾ ਪੈਂਦਾ ਹੈ।
5. ਪੰਜਾਬ …………………………… ਕਰਕੇ ਵੀ ਬਹੁਤ ਪ੍ਰਸਿੱਧ ਹੈ।
6. ਭਾਰਤ ਦਾ ਰਾਸ਼ਟਰੀ ਗਾਣ ‘ਜਣ ਗਣ ਮਨ’ …………………………… ਨੇ ਲਿਖਿਆ ਹੋਇਆ ਹੈ।
7. ਕਿਸੇ …………………………… ਨੂੰ ਨਾਲ ਲੈ ਕੇ ਚਲਨਾ ਹੀ ਅਸਲੀ ਨੇਕੀ ਹੈ।
ਉੱਤਰ :
1. ਪੰਜਾਬ
2. ਬਹਾਦਰੀ
3. ਦਸਵੇਂਗਿਆਰਵੇਂ
4. ਸਰਹੱਦੀ
5. ਖੇਤੀ-ਬਾੜੀ
6. ਰਾਬਿੰਦਰ ਨਾਥ ਟੈਗੋਰ
7. ਕਮਜ਼ੋਰ।

ਸਹੀ/ਗ਼ਲਤ ਦਾ ਨਿਸ਼ਾਨ ਲਗਾਓ :
1. ਪੰਜਾਬ ਦਾ ਨਾਮ ਸੱਤ ਦਰਿਆਵਾਂ ਤੋਂ ਪਿਆ
2. ਪੰਜਾਬ ਸਰਹੱਦੀ ਰਾਜ ਹੋਣ ਕਾਰਨ ਇਥੇ ਸਦਾ ਹੀ ਸ਼ਾਂਤੀ ਬਣੀ ਰਹਿੰਦੀ ਹੈ।
3. ਪੰਜਾਬ ਦੀ ਬੋਲੀ ਡੋਗਰੀ ਹੈ।
4. ਨੱਚਣਾ ਗਾਉਣਾ ਪੰਜਾਬੀਆਂ ਦੀ ਰੂਹ ਦੀ ਖ਼ੁਰਾਕ ਹੈ।
5. ਪੰਜਾਬ ਵਿਚ ਕਣਕ, ਚੌਲ, ਮੱਕੀ ਅਤੇ ਗੰਨੇ ਦੀ ਭਰਪੂਰ ਫ਼ਸਲ ਹੁੰਦੀ ਹੈ।
6. ਭਾਰਤੀ ਰੁਪਏ ਉਪਰ 17 ਭਾਸ਼ਾਵਾਂ ਵਿਚ ਨੋਟ ਰਾਸ਼ੀ ਲਿਖੀ ਮਿਲਦੀ ਹੈ।
7. ਮੁਨਸ਼ੀ ਪ੍ਰੇਮ ਚੰਦ ਹਿੰਦੀ ਦੇ ਬਹੁਤ ਵੱਡੇ ਲੇਖਕ ਸਨ।
8. ਸਾਡੇ ਵਿਚ ਊਚ-ਨੀਚ ਦੀ ਭਾਵਨਾ ਨਹੀਂ ਹੋਣੀ ਚਾਹੀਦੀ ਹੈ।
ਉੱਤਰ :
1. ਗਲਤ
2. ਗਲਤ
3. ਗਲਤ
4. ਠੀਕ
5. ਠੀਕ
6. ਠੀਕ
7. ਠੀਕ
8. ਠੀਕ।
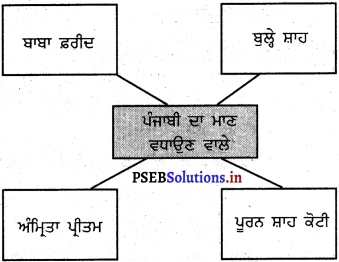
ਮਾਈਂਡ ਮੈਪਿੰਗ :
ਉੱਤਰ :
ਮਿਲਾਨ ਕਰੋ :
1. ਪੰਜਾਬ (ਉ) ਟੈਗੋਰ,
2. ਪੰਜਾਬ ਦਾ ਮੇਲਾ (ਅ) ਸੁਰਜੀਤ ਪਾਤਰ
3. ਪੰਜਾਬੀ ਕਵੀ (ਇ) ਸਰਹੱਦੀ ਰਾਜ
4. ਬੰਗਾਲੀ ਲੇਖਕ (ਸ) ਜਰਗ ਦਾ
ਉੱਤਰ :
1. (ਇ)
2. (ਸ)
3. (ਅ)
4. (ੳ)

ਛੋਟੇ ਉੱਤਰਾਂ ਵਾਲੇ ਪ੍ਰਸ਼ਨ :
ਪ੍ਰਸ਼ਨ 1.
ਪੰਜਾਬ ਦਾ ਨਾਂ ਕਿਹੜੇ ਪੰਜ ਦਰਿਆਵਾਂ ਤੋਂ ਪਿਆ ?
ਉੱਤਰ :
ਸਤਲੁਜ, ਬਿਆਸ, ਰਾਵੀ, ਚਨਾਬ ਅਤੇ ਜਿਹਲਮ।
ਪ੍ਰਸ਼ਨ 2.
ਅੱਜ-ਕਲ੍ਹ ਪੰਜਾਬ ਵਿਚ ਕਿੰਨੇ ਦਰਿਆ ਹਨ ?
ਉੱਤਰ :
ਤਿੰਨ : ਸਤਲੁਜ, ਬਿਆਸ ਅਤੇ ਰਾਵੀ।
ਪ੍ਰਸ਼ਨ 3.
ਪੰਜਾਬ ਵਿਚਲੇ ਦੋ ਦਰਿਆ ਕਿਵੇਂ ਘੱਟ ਗਏ ?
ਉੱਤਰ :
1947 ਦੀ ਵੰਡ ਵਿਚ ਦੋ ਦਰਿਆ ਪਾਕਿਸਤਾਨ ਵਿਚ ਚਲੇ ਗਏ।
ਪ੍ਰਸ਼ਨ 4.
ਪੰਜਾਬ ਦੇ ਲੋਕ ਨਾਚ ਕਿਹੜੇ ਹਨ ?
ਉੱਤਰ :
ਮਰਦਾਂ ਦਾ ਭੰਗੜਾ ਅਤੇ ਔਰਤਾਂ ਦਾ ਨਾਚ ਗਿੱਧਾ ਹੈ।
ਪ੍ਰਸ਼ਨ 5.
ਪੰਜਾਬ ਦੇ ਸ਼ਹੀਦਾਂ ਦੇ ਨਾਮ ਦੱਸੋ।
ਉੱਤਰ :
ਸ਼ਹੀਦ ਭਗਤ ਸਿੰਘ, ਸ਼ਹੀਦ ਕਰਤਾਰ ਸਿੰਘ ਸਰਾਭਾ, ਸ਼ਹੀਦ ਊਧਮ ਸਿੰਘ

ਪ੍ਰਸ਼ਨ 6.
ਪੰਜਾਬ ਦੀ ਬੋਲੀ ਕਿਹੜੀ ਹੈ ?
ਉੱਤਰ :
ਪੰਜਾਬ ਦੀ ਬੋਲੀ ਪੰਜਾਬੀ ਹੈ।
ਪਸ਼ਨ 7.
ਪੰਜਾਬ ਦੀ ਬੋਲੀ ਸੰਸਾਰ ਵਿਚ ਕਿੰਨੇ ਨੰਬਰ ‘ਤੇ ਹੈ ?
ਉੱਤਰ :
ਪੰਜਾਬੀ ਬੋਲਣ ਵਾਲਿਆਂ ਦੀ ਗਿਣਤੀ ਦੇ ਆਧਾਰ ‘ਤੇ ਇਹ ਸੰਸਾਰ ਵਿਚ ਦਸਵੇਂ-ਗਿਆਰਵੇਂ ਨੰਬਰ ਦੀ ਭਾਸ਼ਾ ਹੈ।
ਪ੍ਰਸ਼ਨ 8.
ਮਾਂ ਬੋਲੀ ਕੀ ਹੁੰਦੀ ਹੈ ?
ਉੱਤਰ :
ਮਾਂ ਬੋਲੀ ਉਹ ਬੋਲੀ ਹੁੰਦੀ ਹੈ ਜੋ ਬੱਚਾ ਸ਼ੁਰੂ ਤੋਂ ਹੀ ਆਪਣੇ ਮਾਤਾ-ਪਿਤਾ ਜਾਂ ਪਰਿਵਾਰ ਤੋਂ ਬੋਲਣਾ ਸਿੱਖਦਾ ਹੈ।
ਪ੍ਰਸ਼ਨ 9.
ਕੋਈ ਦੋ ਗੀਤ ਜਿਨ੍ਹਾਂ ਵਿਚ ਮਾਂ ਬੋਲੀ ਬਾਰੇ ਦੱਸਿਆ ਹੈ, ਕਿਹੜੇ ਹਨ ?
ਉੱਤਰ :
ਹਰਭਜਨ ਮਾਨ ਦਾ ਮੈਨੂੰ ਇਉਂ ਨਾ ਮਨੋਂ ਵਿਸਾਰ, ਵੇ ਮੈਂ ਤੇਰੀ ਮਾਂ ਦੀ ਬੋਲੀ ਹਾਂ। ਸਤਿੰਦਰ ਸਰਤਾਜ ਦਾ- ਮੈਂ ਗੁਰਮੁਖੀ ਦਾ ਬੇਟਾ।
ਪ੍ਰਸ਼ਨ 10.
ਗੁਰਮੁਖੀ ਕੀ ਹੈ ?
ਉੱਤਰ :
ਇਹ ਪੰਜਾਬੀ ਭਾਸ਼ਾ ਦੀ ਲਿਪੀ ਹੈ।

ਪ੍ਰਸ਼ਨ 11.
ਮਨਮੋਹਨ ਵਾਰਿਸ ਦੇ ਗੀਤ ਅਨੁਸਾਰ ਕਿਹੜੀਆਂ ਤਿੰਨ ਮਾਂਵਾਂ ਹੁੰਦੀਆਂ ਹਨ ?
ਉੱਤਰ :
ਧਰਤੀ ਮਾਂ, ਮਾਂ-ਬੋਲੀ, ਜਨਮ-ਦਾਤੀ ਮਾਂ।

![]()