Punjab State Board PSEB 11th Class Chemistry Important Questions Chapter 1 Some Basic Concepts of Chemistry Important Questions and Answers.
PSEB 11th Class Chemistry Important Questions Chapter 1 Some Basic Concepts of Chemistry
Very Short Answer Type Questions
Question 1.
A liquid has a volume of 49.0 cm3 and a mass of 57.642 g. Find out the density of this liquid in SI unit.
Answer:
Density = \(\frac{\text { Mass }(\mathrm{kg})}{\text { Volume }\left(\mathrm{m}^{3}\right)}\)
Mass = 57.642 g = 57.642 x 10-3 kg
Volume = 49.0 cm3 = 49.0 x (10-3)3 = 49.0 x 10-6 m3
∴ Density = \(\frac{57.642 \times 10^{-3}}{49.0 \times 10^{-6}}\) = 1.176 x 103 kg/m3
![]()
Question 2.
Convert the following temperatures into degree Fahrenheit,
(i) 25°C, physiological (human body) temperature.
(ii) 35°C, the room temperature.
Answer:
(i) Given
C = 25 °C
°F = \(\frac{9}{5}\) °C + 32 = \(\frac{9}{5}\) x 25 + 32 = 45 + 32 = 77°F
(ii) Given, C = 35 °C
°F = \(\frac{9}{5}\) °C + 32 = \(\frac{9}{5}\) x 35 + 32 = 63 + 32 = 95°F
Question 3.
What is the mass (in grams) of a copper block whose dimensions are 5.0 inch x 6.0 inch and whose density is 8.96 g/cm3? Given that 1 inch = 2.54 cm
Answer:
Here, unit conversion factors are 1 = \(\frac{2.54 \mathrm{~cm}}{1 \text { inch }}=\frac{1 \text { inch }}{2.54 \mathrm{~cm}}\)
Hence, required mass (in g) = 5.0 inch x 6.0 inch x 4.0 inch
\(\) = 1.76 x 104 g.
Question 4.
If 6.3 g of NaHCO3 are added to 15.0 g of CH3COOH solution, the residue is found to weigh 18.0 g. What is the mass of CO2 released in the reaction?
Answer:
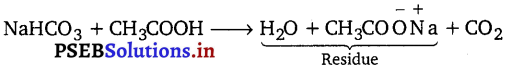
Sum of the masses of reactants = 6.3 + 15 = 21.3 g
Sum of the masses of products = x + 18
21.3 = x +18; x = 21.3 – 18 = 3.3 g
Thus, the mass of the C02 released is 3.3 g.
Question 5.
Why is the law of Gay Lussac’s not obeyed if any reactant or product is not a gas?
Answer:
If any reactant or product is a liquid or solid, the volume occupied by them is extremely small as compared to the gas and hence, the law is not obeyed.
Question 6.
Calculate the normality of solution containing 62.3 g of hydrated copper sulphate (CuSO4 . 5H2O) in 500 mL of solution.
Answer:
Mass of solute = 62.3 g
Equivalent mass of oxalic acid = \(\frac{249.5}{2}\) = 124.75 g
Gram equivalents of oxalic acid = \(\frac{62.3}{124.75}\) = 0.5
Volume of solution = 500 mL
Normality = \(\frac{0.5}{500}\) x 1000 = 1N
![]()
Question 7.
What is the difference between molality and molarity?
Answer:
Molality : It is defined as the number of moles of solute dissolved in 1 kg of solvent. It is independent of temperature. ,
Molarity : It is defined as the number of moles of solute dissolved in 1 L of solution. It depends upon temperature because, volume of solution °c temperature.
Question 8.
How is the term material different from matter?
Answer:
Anything which has mass and occupies space is called matter. However, material corresponds to the matter which has specific use.
Question 9.
Why are the atomic masses of most of the elements fractional?
Answer:
It is because most of the elements occur in nature as a mixture of isotopes and their atomic masses are the average relative atomic masses of the isotopes depending on their abundance.
Question 10.
Volume of a solution changes with change in temperature, then, will the molality of the solution be affected by temperature? Give reason for your answer.
Answer:
No, molality of solution does not change with temperature since mass remains unaffected with temperature.
Short Answer Type Questions
Question 1.
Express the following in the scientific notation.
(i) 0.000968
(ii) 157428
(iii) 90,000
(iv) (5.7 x 106) x (4.2 x 10-2)
(v) (6.8 x 10-9) + (1.4 x 10-6)
(vi) (456 x 103 + 2.62 x 102)
(vii) (9.87 x 10-3 – 2.26 x 10-4)
Answer:
(i) 0.000968 = 9.68 x 10-4
(ii) 157428 = 1.57428 x 105
(iii) 90,000 = 9 x 104
(iv) (5.7 x 106)x (4.2 x 10-2) = (5.7 x 4.2)(106-2)
= 23.94 x 104 =2.394 x 105
(v) (6.8 x 10-9) ÷ (1.4 x 10-6) = x (10-9-(-6)) = 4.857 x 10-3
(vi) (4.56 x 103 + 2.62 x 102)= 45.6 x 102 + 2.62 x 102
=(45.6 +2.62) x 102
= 48.22 x 102
= 4.822 x 103
(vii) (9.87 x 10-3 — 2.26 x 10-4) = 9.87 x 10-3 — 0.226 x 10-3
= (9.87 – 0.226) x 10-3 = 9.644 x 10-3
![]()
Question 2.
The percentage composition of elements in NH3, H20, and N20 3 is given below. –
NH3 → 82.35% N and 17.65% H
H2O → 88.90% O and 11.10% H
N2O3 → 6a 15% O and 36.85% N
Show that these data are in accordance with the law of reciprocal proportion.
Answer:
(i) In NH3, 1 part of H reacts with = \(\) = 4.67 part of N
(ii) In H2O, 1 part of H reacts with = \(\) = 8.01 part of O
Thus, the ratio N : O :: 4.67 : 8.01 = 1 :1.72
(iii) In N2O3, N and O reacts with each other in the ratio N : O :: 36.85: 63.15 = 1 :1.71.
Thus, the two ratios are the same. Hence, it illustrates the law of reciprocal proportions.
Question 3.
A solution contains 25% water, 25% ethanol and 50% acetic acid by mass. Calculate the mole fraction of each component.
Answer:
Let the total mass of solution = 100 g
Mass of water = 25 g, Mass of ethanol = 25 g
Mass of acetic acid = 50 g
Moles of water = \(\frac{25}{18}\) = 1.388 (∵ Molar mass of H2O= 18)
Moles of ethanol = \(\frac{25}{46}\) = 0.543 (∵ Molar mass of C2H5OH = 18)
Moles of acetic acid = \(\frac{50}{60}\) = 0.833 (∵ Molar mass of CH3COOH = 18)
Total number of moles = 1.388 + 0.543 + 0.833 = 2.764
Mole fraction of water = \(\frac{1.388}{2.764}\) = 0.502
Mole fraction of ethanol = \(\frac{0.543}{2.764}\) = 0.196
Mole fraction of acetic acid = \(\frac{0.833}{2.764}\) = 0.302
Question 4.
Calculate the molality of a solution containing 20.7 g potassium carbonate dissolved in 500 mL of solution (assume density of solution = 1 g mL-2).
Mass of K2CO3 = 20.7 g
Molar mass of K2CO3 = 2 x 39 + 12 + 3 x 16 = 138 mol-1
Moles of K2CO3 = \(\frac{20.7}{138}\) = 0.15 .
Mass of solution = (500 mL) x (1 g mL-1) = 500 g
Amount of water = 500 – 20.7 = 479.3 g
Molality = \(\frac{Moles of solute}
{Mass of solvent in gram}\) x 100
= \(\frac{0.15}{479.3}\) x 1000 = 0.313 mx`
Question 5.
45.4 L of dinitrogen reacted with 22.7 L of dioxygen and 45.4 L of nitrous oxide was formed. The reaction is given below 2N2(g) + O2(g) → 2N2O(g)
Which law is being obeyed in this experiment? Write the statement of the law. [NCERT Exemplar]
Answer:
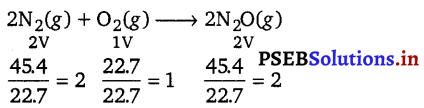
Hence, the ratio between the volumes of the reactants and the product in the given question is simple i.e., 2:1:2. It proves the Gay-Lussac’s law of gaseous volumes.
Gay-Lussac’s law of gaseous volumes : The law of combining volume states that when gases react together to form other gases, and when all volumes are measured at the same temperature and pressure, the ratio between the volumes of the gaseous reactants and products can be expressed in simple whole numbers.
Long Answer Type Questions
Question 1.
Copper oxide was prepared by the following methods.
(i) In first case, 1.75 g of the metal was dissolved in nitric acid and igniting the residual copper nitrate yielded 2.19 g of copper oxide.
(ii) In the second case, 1.14 g of metal dissolved in nitric acid were precipitated as copper hydroxide by adding caustic alkali solution. The precipitated copper hydroxide after washing, drying and heating yielded 1.43 g of copper oxide.
(iii) In the third case, 1.46 g of copper when strongly heated in a current of air yielded 1.83 g of copper oxide. Show that the given data illustrate the law of definite composition.
Answer:
Step I and II In the first experiment,
2.19 g of copper oxide contained 1.75 g of Cu.
∴ 100 g of copper oxide contained Cu
i.e., %ofCu = 79.91
In the second experiment,
1.43 g of copper oxide contained 1.14 g copper.
∴ 100 g of copper oxide contained Cu = \(\frac{1.14}{1.43}\) x 100 = 79.72 g,
i.e„ % of Cu = 79.72
In the third experiment,
1.83 g of copper oxide contained 1.46 g of copper
∴ 100 g of copper oxide contained Cu = \(\frac{1.46}{1.83}\) x 100 = 79.78g.
i.e., % of Cu = 79.78
Step III The percentage of copper in copper oxide derived from all the three experiments is nearly the same. Hence, the above data illustrate the law of definite composition.
![]()
Question 2.
Three oxides of nitrogen contained 63.6%, 46.7% and 30.4% nitrogen respectively. Show that these figures illustrate the law of multiple proportions.
Answer:
In case first,
Step I The oxide of nitrogen contains 63.6% N ‘ i.e., 63.6 g of N reacts with (100 – 63.6) g of O = 36.4 g of O.
Step II ∴ 1 g of N will react with \(\frac{36.4}{63.6}\) g of O = 0.57 g of O.
In case second,
Step I The oxide of nitrogen contains 46.7% N
i.e., 46.7 g of N reacts with (100 – 46.7) g of O = 53.3 g of O.
In case third,
Step I The oxide of nitrogen contains 30.4% N
i.e., 30.4 g of N reacts with (100 – 30.4) g of O = 69.6 g of O.
Step II ∴ 1 g of N will react with \(\frac{69.6}{30.4}\) of O = 2.26 g of O
Step III This means the ratio of the masses of oxygen which combine with 1 g of nitrogen is 0.57 : 1.14 : 2.26, i.e., 1: 2 : 4 is obviously in accordance with the law of multiple proportions.
