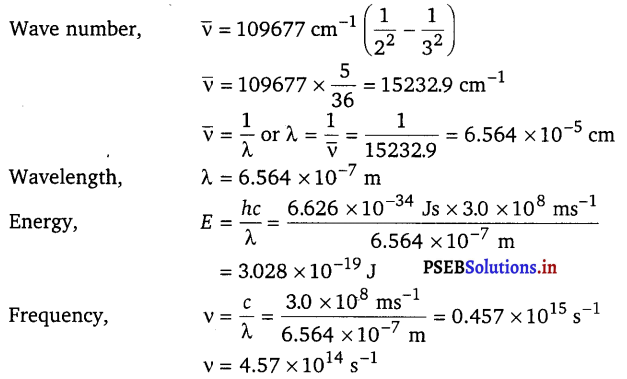Punjab State Board PSEB 11th Class Chemistry Important Questions Chapter 2 Structure of Atom Important Questions and Answers.
PSEB 11th Class Chemistry Important Questions Chapter 2 Structure of Atom
Very Short Answer Type Questions
Question 1.
Which of the following will not show deflection from the path on passing through an electric field? Proton, cathode rays, electron, neutron
Answer:
Neutron is a neutral particle. Hence, it will not be deflected on passing through an electric field.
Question 2.
What is the nuclear radius of an atom whose mass number is 125?
Answer:
Nuclear radius, r = R0A1/3 where, R0 = 1.4 x 10-15 m,
∴ r = (1.4 x 10-15 m) x (125)1/3 = 7.0 x 10-15 m.
![]()
Question 3.
The magnitude of charge on the electron is 4.8 x 10-10 esu. What is the charge on the nucleus of a helium atom?
Answer:
Helium nucleus contains 2 protons and charge of a proton is same as that of an electron.
Therefore, the charge on the nucleus of a helium atom is (+2) x 4.8 x 10-10 = + 9.6 x 10-10 esu.
Question 4.
What is the difference in the origin of cathode rays and anode rays?
Answer:
Cathode rays originate from the cathode whereas anode rays do not originate from the anode. They are produced from the gaseous atoms by knock out of the electrons with high speed cathode rays.
Question 5.
What is the difference between atomic mass and mass number?
Answer:
Mass number is a whole number because it is the sum of number of protons and number of neutrons whereas atomic mass is fractional because it is the average relative mass of its atom as compared with mass of an atom of C-12 isotope taken as 12.
Question 6.
What is the difference between a quantum and a photon?
Answer:
The smallest packet of energy of any radiation is called a quantum whereas that of light is called photon.
Question 7.
Arrange s, p and rf-subshells of a shell in the increasing order of , effective nuclear charge (Zeff) experienced by the electron present in them. [NCERT Exemplar]
Ans. s-orbital is spherical in shape, it shields the electrons from the nucleus more effectively than p-orbital which in turn shields more effectively than d-orbital. Therefore, the effective nuclear charge (Zeff) experienced by electrons present in them is d < p < s.
Question 8.
Show the distribution of electrons in oxygen atom (atomic number 8) using orbital diagram.
Answer:
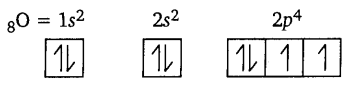
Question 9.
Nickel atom can lose two electrons to form Ni ion. The atomic number of Ni is 28. From which orbital will nickel lose two electrons? [NCERT Exemplar]
Answer:
28Ni = 1s2, 2s2, 2p6, 3s2, 3p6, 3d8, 4s2; Nickel will lose 2 electrons from 4s (outer most shell) to form Ni2+ ion.
Question 10.
Which of the following orbitals are degenerate?
3dxy, 4dxy, \(3 d_{z^{2}}\), 3dyz, \(4 d_{z^{2}}\)
Answer:
The orbitals which belongs to same subshell and same shell are called degenerate orbitals. (3dxy, \(3 d_{z} 2\), 3dyz) and (4dxy, 4dyz, 4d 2) are the two sets of degenerate orbitals.
![]()
Short Answer Type Questions
Question 1.
The Balmer series in the hydrogen spectrum corresponds to the transition from n1 = 2 to n2 = 3, 4 … . This series lies in the visible region. Calculate the wave number of line associated with the transition in Balmer series when the electron moves to n = 4 orbit. (RH = 109677 cm1)
Answer:
From Rydberg formula,
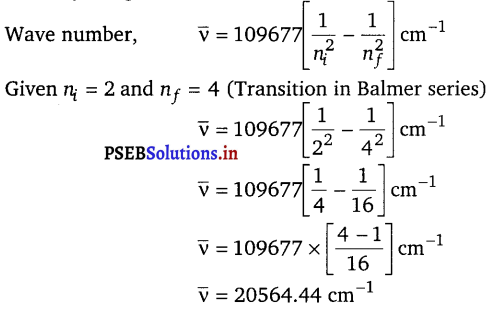
Question 2.
Out of electron and proton which one will have, a higher velocity to produce matter waves of the same wavelength? Explain it.
Answer:
From de Broglie equation, wavelength, \(\lambda=\frac{h}{m v}\)
For same wavelength for two different particles, i.e., electron and proton, m1v1 = m2v2 (h is constant). Lesser the mass of the particle, greater will be the velocity. Hence, electron will have higher velocity.
Question 3.
Wavelengths of different radiations are given helow.
λ(A) = 300 nm, λ(B) = 300 pm, λ(C) = 3 nm, λ(D) = 30Å Arrange these radiations in the increasing order of their energies.
Answer:
(A) λ=3OOnm=3OO x 10-9m
(B) λ =300µm=300 x 10-6m
(C) λ =3nm = 3 x 10-9 m
(D) λ = 30 = 30 x 10-10m= 3 x 10-9m
Energy, E = \(\frac{h c}{\lambda}\)
Therefore, E ∝ \(\frac{1}{\lambda}\)
Increasing order of energy is B
Question 4.
The electronic configuration of valence shell of Cu is 3d104s1 and not 3d94s2. How is this configuration explained?
Answer:
Configurations with completely filled and half-filled orbitals have extra stability. In 3d104s1, d-orbitals are completely filled and s-orbital is half-filled. Hence, it is a more stable configuration for Cu as compare to 3d94s2.
Question 5.
In each of the following pairs of salts, which one is more stable? (i) Ferrous and ferric salts (ii) Cuprous and cupric salts
Answer:
(i) Ferrous and ferric salts : In ferrous salts Fe2+, the configuration is 1s2,2s2,2p6,3s2,3p6,3d6. In ferric salts Fe3+, the configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 3d5. As half-filled 3d5 configuration is more stable therefore ferric salts are more stable than ferrous salts.
(ii) Cuprous and cupric salts : In cuprous salts, the configuration of Cu+ is 1s2,2s2, 2p6, 3s2, 3p6, 3d10. In cupric salts the configuration of Cu2+, is, 1s2,2s2,2p6,3s2,3p6,3d9. Although Cu+ has completely filled d-orbital, yet cuprous salts are less stable. This is because the nuclear charge is not sufficient enough to hold 18 electrons of Cu+ ion present in the outermost shell.
Long Answer Type Questions
Question 1.
When an electric discharge is passed through hydrogen gas, the hydrogen molecules dissociate to produce excited hydrogen atoms. These excited atoms emit electromagnetic radiation of discrete frequencies which can be given by the general formula \(\bar{v}=109677\left[\frac{1}{n_{i}^{2}}-\frac{1}{n_{f}^{2}}\right]\)
What points of Bohr’s model of an atom can he used to arrive at this formula? Based on these points derive the above formula giving description of each step and each term. [NCERT Exemplar]
Answer:
The two important points of Bohr’s model that can be used to derive the given formula are as follows :
(i) Electrons revolve around the nucleus in a circular path of fixed radius and energy. These paths are called orbits, stationary states or allowed energy states.
(ii) Energy is emitted or absorbed when an electron moves from higher stationary state to lower stationary state or from lower stationary state to higher stationary state respectively.
Derivation : The energy of the electron in the nth stationary state is given by the expression,
\(E_{n}=-R_{\mathrm{H}}\left(\frac{1}{n^{2}}\right)\)
n = 1,2,3 …. ……(i)
where RH is called Rydberg constant and its value is 2.18 x 10-18 J.
The Energy of the lowest state, also called the ground state, is
E1 = -2.18 x 10-18\(\left(\frac{1}{1^{2}}\right)\) = 2.18 x 10-18J ……. (ii)
The energy gap between the two orbits is given by the equation,
ΔE = Ef – Ei … (iii)
On combining equations (i) and (iii)
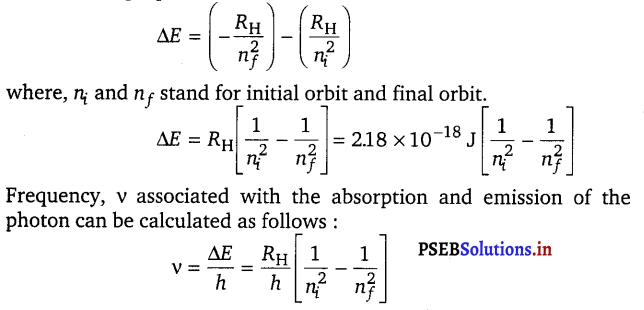
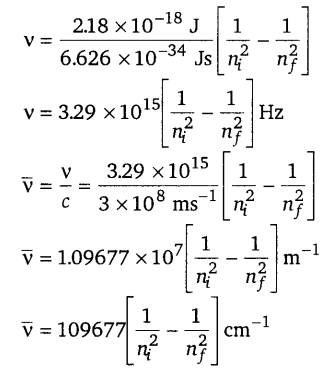
![]()
Question 2.
Calculate the energy and frequency of the radiation emitted when an electron jumps from n = 3 to n = 2 in a hydrogen atom.
Answer: