Punjab State Board PSEB 11th Class Chemistry Important Questions Chapter 6 Thermodynamics Important Questions and Answers.
PSEB 11th Class Chemistry Important Questions Chapter 6 Thermodynamics
Very Short Answer Type Questions
Question 1.
Identify the state functions and path functions out of the following.
Enthalpy, entropy, heat, temperature, work, free energy.
Answer:
State function Enthalpy, entropy, temperature, free energy.
Path function Heat, work
Question 2.
At 1 atm will the ΔfH0 be zero for Cl2(g) and Br2(g)? Explain.
Answer:
ΔfH0 for Cl2(g) will be zero but ΔfH0 for Br2(g) will not be zero because liquid bromine state is elementary state not gaseous.
![]()
Question 3.
Why for predicting the spontaneity of a reaction, free energy criteria is better than the entropy criteria?
Answer:
Criteria of free energy change is better because it requires free energy change of the system only whereas the entropy change requires the total entropy change of the system and the surroundings.
Question 4.
Water can be lifted into the water tank at the top of the house with the help of a pump. Then why is it not considered to be spontaneous?
Answer:
A spontaneous process should occur continuously by itself after initiation. But this is not so in the given case because water will go up so long as the pump is working.
Question 5.
Given that ΔH = 0 for mixing of two gases. Explain whether the diffusion of these gases into each other in a closed container is a spontaneous process or not?
Answer:
It is a spontaneous process because although ΔH = 0, i.e., energy factor has no role to play but randomness increases, i.e., randomness factor favours the process.
Question 6.
Under what condition, the heat evolved or absorbed in a reaction is equal to its free energy change?
Answer:
As ΔG = ΔH – TΔS. Thus, ΔG = ΔH only when either the reaction is carried out at 0 K or the reaction is not accompanied by any entropy change, i.e., ΔS = 0.
Question 7.
In the equation, N2(g) + 3H2(g) ⇌ 2NH3(g) what would be the sign of work done?
Answer:
The sign of work done will be positive, i.e., work will be done on the system due to decrease in volume.
Question 8.
The molar enthalpy of vaporisation of acetone is less than that of water. Why?
Answer:
Enthalpy of vaporisation of water is more than that of acetone because there is strong hydrogen bonding in H2O molecules.
![]()
Question 9.
One mole of acetone requires less heat to vaporise than 1 mole of water. Which of the two liquids has higher enthalpy of vaporisation?
Answer:
Less the heat required to vaporise 1 mole of a liquid, less is its enthalpy of vaporisation. Hence, water has higher enthalpy of vaporisation.
Question 10.
Which quantity out of ΔrG and ΔrG° will be zero at equilibrium?
Answer:
ΔrG = ΔrG° + RT In K
At equilibrium, 0 (zero) = ΔrG° + RT In K
(v ΔrG = 0)
or ΔrG° = -RT In It;
ΔrG° = 0 when it = 1
For all other values of K, ΔrG° will be non-zero.
Short Answer Type Questions
Question 1.
Define the following :
(i) First law of thermodynamics.
(ii) Standard enthalpy of formation.
Answer:
(i) First law of thermodynamics : It states that energy can neither be created nor be destroyed. The energy of an isolated system is constant. ΔU = q + w
(ii) Standard Enthalpy of Formation : It is defined as the amount of heat evolved or absorbed when one mole of the compound is formed from its constituent elements in their standard states.
Question 2.
Give reason for the following:
(i) Neither q nor w is a state function but q + w is a state function.
(ii) A real crystal has more entropy than an ideal crystal.
Answer:
(a) q + w = ΔU
As ΔU is a state function hence, q + w is a state function.
(b) A real crystal has some disorder due to the presence of defects in its structural arrangement whereas ideal crystal does not have any disorder. Hence, a real crystal has more entropy than an ideal crystal.
![]()
Question 3.
Represent the potential energy/enthalpy change in the following processes graphically
(i) Throwing a stone from the ground to roof.
(ii) \(\frac{1}{2}\)H2(g) + \(\frac{1}{2}\)Cl2(g) ⇌ HCl(g); ΔrHs = – 92.32kJ mol-1
In which of the processes potential energy/enthalpy change is contributing factor to the spontaneity?
Answer:
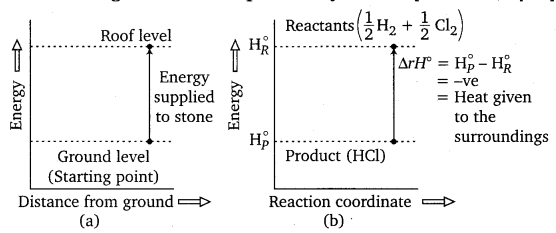
Energy increases in (a) and it decreases in (b). Hence, in process (b), enthalpy change is the contributing factor to the spontaneity.
Question 4.
A man takes a diet equivalent to 10000 kJ per day and does work, by expending his energy in all forms equivalent to 12500 kJ per day. What is change in internal energy per day? If the energy lost was stored as sucrose (1632 kJ per 100 g), how many days should it take to lose 2 kg of his weight? (Ignore water loss)
Answer:
Energy taken by a man = 10000 kJ
Change in internal energy per day = 12500 -10000 = 2500 kJ
The energy is lost by the man as he expends more energy than he takes.
Now 100 g of sugar corresponds to energy = 1632 kJ loss in energy.
2000 g of sugar corresponds to energy = \(\frac{1632 \times 2000}{100}\) = 32640 kJ
∴ Number of days required to lose 2000 g of weight or 32640 kJ of energy = \(\frac{32640}{2500}\) = 13 days
Question 5.
Give the appropriate reason :
(i) It is preferable to determine the change in enthalpy rather than the change in internal energy.
(ii) It is necessary to define the ‘standard state’.
(iii) It is necessary to specify the phases of the reactants and products in a thermochemical equation.
Answer:
(i) Because it is easier to make measurement under constant pressure than under constant volume conditions.
(ii) Enthalpy change depends upon the conditions in which a reaction is carried out. For making the comparison of results obtained by different people meaningful, the reaction conditions must be well-defined.
(iii) Because enthalpy depends upon the phase of reactants and products.
Long Answer Type Questions
Question 1.
(i) A cylinder of gas supplied by a company is assumed to contain 14 kg of butane. If a normal family requires 20000 kJ of energy per day for cooking, how long will the cylinder last?
(ii) If the air supplied to the burner is insufficient, a portion of gas escapes without combustion. Assuming that 25% of the gas is wasted due to this inefficiency, how long will the cylinder last (Heat of combustion of butane = 2658kJ!mol.)?
Answer:
(i) Molecular formula of butane = C4H10
Molecular mass of butane = 4 x 12 +10 x 1 = 58
Heat of combustion of butane 2658 kJ mol-1
1 mole.or 58 g of butane on complete combustion gives heat = 2658 kJ
∴ 14 x 103 g of butane on complete combustion will give heat
= \(\frac{2658 \times 14 \times 10^{3}}{58}\) = 641586 kJ
The family needs 20000 kJ of heat per day.
∴ 20000 kJ of heat is used for cooking by a family in 1 day.
∴ 641586 kJ of heat will be used for cooking by a family in
= \(\frac{641586}{20000}\) = 32days
The cylinder will last for 32 days
(ii) 25 per cent of the gas is wasted due to inefficiency. This means that only 75% of butane gets combusted. Therefore, the energy produced by
75% combustion of butane = \(\frac{641586 \times 75}{100}\) = 481189.5 kJ
∴ The number of days the cylinder will last = \(\frac{481189.5}{20000}\) = 24 days.
![]()
Question 2.
10 moles of an ideal gas expand isothermally and reversibly from a pressure of 5 atm to 1 atm at 300 K. What is the largest mass that can be lifted through a height of 1 m by this expansion?
Answer:
Wexp = -2.303 nRT log \(\frac{p_{1}}{p_{2}}\)
= -2.303(10) x (8.314)(300) log \(\frac{5}{1}\) = – 40.15 x 103 J
If M is the mass that can be lifted by this work through a height of 1 m, then work done = Mgh
40.15 x 103 J = M x 9.81 ms-1 x 1 m
or M = \(\frac{40.15 \times 10^{3} \mathrm{~kg} \mathrm{~m}^{2} \mathrm{~s}^{-2}}{9.81 \mathrm{~m} \mathrm{~s}^{-2} \times 1 \mathrm{~m}}\) [∵ J = kg m2s-2]
= 4092.76 kg
