Punjab State Board PSEB 11th Class Chemistry Book Solutions Chapter 14 Environmental Chemistry Textbook Exercise Questions and Answers.
PSEB Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
PSEB 11th Class Chemistry Guide Environmental Chemistry InText Questions and Answers
Question 1.
Define environmental chemistry.
Answer:
Environmental chemistry is the study of chemical and biochemical processes occurring in nature. It deals with the study of origin, transport, reaction, effects, and fates of various chemical species in the environment.
![]()
Question 2.
Explain tropospheric pollution in 100 words.
Answer:
Tropospheric pollution occurs due to the presence of undesirable solid or gaseous particles in the air. The major gaseous and particulate pollutants present in the troposphere are :
(i) Gaseous air pollutants : These are oxides of sulphur, nitrogen and carbon, hydrogen sulphide, hydrocarbons, ozone and other oxidants.
(ii) Particulate pollutants : These are dust, mist, fumes, smoke, smog, etc.
Gaseous Air Pollutants
(a) Oxides of sulphur : These are produced when sulphur containing fossil fuel is burnt. S02 gas is poisonous to both animals and plants.
(b) Oxides of nitrogen : These are produced by the reaction of nitrogen and oxygen at high altitudes when lightning strikes.
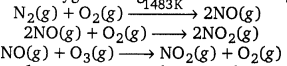
(c) Hydrocarbons : Incomplete combustion of fuel used in automobiles is the major source for the release of hydrocarbon. These are carcinogenic and cause cancer. They also harm plants.
(d) Oxides of carbon : Carbon monoxide is one of the most serious air pollutants. It is highly poisonous to living beings because it blocks the supplyof oxygen to the organs and tissues. It is produced due to the incomplete combustion of carbon.
Carbon dioxide is the main contributor towards green house effect and global warming. It is released into the atmosphere by respiration, burning of fossil fuels and by decomposition of limestone during cement manufacturing.
Question 3.
Carbon monoxide gas is more dangerous than carbon dioxide gas. Why?
Answer:
Carbon dioxide (CO2) and carbon monoxide (CO) gases are emitted during the combustion of various fuels. Carbon monoxide is poisonous, whereas carbon dioxide is non-toxic in nature.
Carbon monoxide is poisonous because it is capable of forming a complex with haemoglobin (carboxyhaemoglobin), which is more stable than the
oxygen-heamoglobin complex. The concentration range (3-4% )of carboxyhaemoglobin decreases the oxygen-carrying capacity of blood. This results in headaches, weak eyesight, nervousness, and cardiovascular disorders. A more increased concentration may even lead to death.
Carbon dioxide is not poisonous. It proves harmful only at very high concentrations.
Question 4.
List gases which are responsible for greenhouse effect.
Answer:
The major greenhouse gases are:
1. Carbon dioxide (CO2)
2. Methane (CH4)
3. Nitrous oxide (NO)
4. Ozone (O3)
5. Chlorofluorocarbons (CFCs)
Question 5.
Statues and monuments in India are affected by acid rain. How?
Answer:
Acid rain is a byproduct of various human activities that leads to the emission of oxides of sulphur and nitrogen in the atmosphere. These oxides undergo oxidation and then react with water vapour to form acids.
2SO2(g) + O2(g) + 2H2O(l) > 2H2SO4(aq)
4NO2(g) + O2(g) + 2H2O(l) > 4HNO3(aq)
Acid rain causes damage to buildings and structures made of stone and metal.
In India, limestone is a major stone used in the construction of various monuments and statues, including the Taj Mahal.
Acid rain reacts with limestone as:
CaCO3 + H2SO4 > CaSO4 + H2O + CO2
This results in the loss of lustre and colour of monuments, leading to their disfiguration.
![]()
Question 6.
What is smog? How is classical smog different from photochemical smog?
Answer:
Smog is a kind of air pollution. It is the blend of smoke and fog. There are two kinds of smog.
(a) Classical smog
(b) Photochemical smog ^
The two smogs can be differentiated as follows :
table
Question 7.
Write down the reactions involved during the formation of photochemical smog.
Answer:
Photochemical smog is formed as a result of the reaction of sunlight with hydrocarbons and nitrogen oxides. Ozone, nitric oxide, acrolein, formaldehyde, and peroxyacetyl nitrate (PAN) are common components of photochemical smog. The formation of photochemical smog can be summarized as follows:
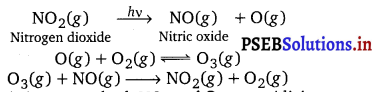
Burning of fossil fuels leads to the emission of hydrocarbons and nitrogen dioxide in the atmosphere. High concentrations of these pollutants in air results in their interaction with sunlight as follows:
![]()
Question 8.
What are the harmful effects of photochemical smog and how can they be controlled?
Answer:
Effects of photochemical smog : Photochemical smog is oxidizing smog owing to the presence of N02 and 03 causing corrosion of metals, stones, rubber, and painted surfaces. The other major components of photochemical smog are PAN, acrolein, and formaldehyde. Both PAN and ozone are eye irritants, while nitric oxide (formed from NO2) causes nose and throat irritation. At higher concentrations, photochemical smog causes chest pain, headaches, throat dryness, and various respiratory ailments.
Control measures : Photochemical smog results from the burning of fossil fuels and automobile fuels that emit NO2 and hydrocarbons, which in turn form ozone, PAN and other chemicals. The use of catalytic converters in automobiles is recommended to prevent the release of NO2 and hydrocarbons into the atmosphere.
Plantation of plants such as Finns, Juniparus, Quercus, Pyrus, and Vitis is also advised as these plants have the capability to metabolize NO2.
Question 9.
What are the reactions involved for ozone layer depletion in the stratosphere?
Answer:
In the stratosphere, ozone is a product of the action of UV radiations on dioxygen as:
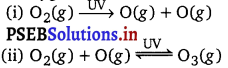
Reaction (ii) indicates the dynamic equilibrium existing between the production and decomposition of ozone molecules. Any factor that disturbs the equilibrium may cause depletion of ozone layer by its decomposition. One such factor is the release of chlorofluorocarbon compounds (CFCs). These are non-reactive, non-flammable molecules that are used in refrigerators, air conditioners, plastics, and electronic industries.
Once released CFCs mix with atmospheric gases and reach the stratosphere, where they are decomposed by UV radiations.
![]()
The chlorine free radical produced in reaction (iii) reacts with ozone as:
![]()
The ![]() radicals further react with atomic oxygen to produce more chlorine radicals as:
radicals further react with atomic oxygen to produce more chlorine radicals as:
![]()
The regeneration of ![]() causes a continuous breakdown of ozone present in the stratosphere damaging the ozone layer.
causes a continuous breakdown of ozone present in the stratosphere damaging the ozone layer.
![]()
Question 10.
What do you mean by ozone hole? What are its consequences? Ans. In Polar regions, stratospheric clouds provide the surface for chlorine nitrate and hypochlorous acid, which react further to give molecular chlorine. Molecular chlorine and H0C1 are photolysed to give chlorine-free radicals.
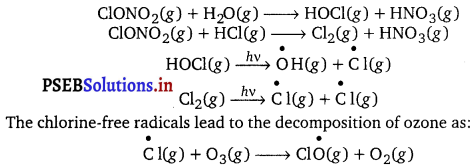
Hence, a chain reaction is initiated. The chlorine-free radical is continuously regenerated, thereby depleting the ozone layer. This phenomenon is known as the ozone hole.
Effects of depletion of ozone layer : The ozone layer protects the Earth from the harmful UV radiations of the sun. With the depletion of the layer, more radiation will enter the Earth’s atmosphere. UV radiations are harmful because they lead to the ageing of skin, cataract, skin cancer, and sunburns. They cause death of many phytoplanktons, which leads to a decrease in fish productivity. Excess exposure may even causes mutation in plants.
Increase in UV radiations, decreases the moisture content of the soil and damages both plants and fibres.
Question 11.
What are the major causes of water pollution? Explain.
Answer:
Several human activities caused water population which leads to the presence of several undesirable substances in water.
Major water pollutants with their sources have been tabulated as follows:
table
Roles played by major pollutants are :
1. Pathogens : These water pollutants include bacteria and other organisms. They enter water from animal excreta and domestic sewage. Bacteria present in human excreta (for example, Escherichia coli and Streptococcus faecalis) cause gastrointestinal diseases.
2. Organic wastes : These are biodegradable wastes that pollute water as a result of run off. The presence of excess organic wastes in water decreases the amount of oxygen held by water. This decrease in the amount of dissolved oxygen inhibits aquatic life.
3. Chemical pollutants : These are water soluble chemicals like heavy metals such as cadmium, mercury, nickel, etc. The presence of these chemicals (above the tolerance limit) can damage the kidneys, central nervous system, and liver.
Question 12.
Have you ever observed any water pollution in your area? What measures would you suggest to control it?
Answer:
Water pollution arises as a result of various human activities. This includes discharges from waste water treatment plants, run-off from agricultural fields, storm water drainage, etc. Pollutants from these sources enter the water bodies, thereby contaminating the water and rendering it impure.
Industries and chemical factories discharge toxic, heavy metals such as Fe, Mn, Al, etc., along with organic wastes into water. Domestic sewage and animal excreta are also responsible for pathogenic contamination of water.
These pollutants make water unfit for drinking.
Therefore, all industrial and chemical discharges should be made free from toxic metals before allowing them to enter a water body. The concentration of these pollutants should be checked regularly. Compost should be preferred over chemical fertilizers in gardens and agricultural fields to avoid harmful chemicals from entering ground water.
Question 13.
What do you mean by Biochemical Oxygen Demand (BOD)?
Answer:
Biochemical oxygen demand is the amount of oxygen required by bacteria to decompose organic matter in a certain volume of sample of water. Clean water would have a BOD value of less than 5 ppm, whereas highly polluted water has a BOD value of 17 ppm or more.
Question 14.
Do you observe any soil pollution in your neighbourhood? What efforts will you make for controlling the soil pollution?
Answer:
Major sources of soil pollution are industrial wastes and agricultural pollutants such as pesticides, fertilizers, etc.
It is very important to maintain the quality and fertility of soil to ensure and sustain the growth of plants and food crops.
Insecticides like DDT are not soluble in water. For this reason, they remain in soil for a long time contaminating the root crops. Pesticides like Aldrin and Dieldrin are non-biodegradable and highly toxic in nature. They can enter the higher trophic levels through food chains, causing metabolic and physiological disorders. The same is true for industrial wastes that comprises of several toxic metals like Pb, As, Hg, Cd, etc.
Hence, the best way to check soil pollution is to avoid direct addition of pollutants to the soil. Also, wastes should undergo proper treatment. They should be recycled and only then, allowed to be dumped. i
![]()
Question 15.
What are pesticides and herbicides? Explain giving examples.
Answer:
Pesticides are a mixture of two or more substances. They are used for killing pests. Pests include insects, plant pathogens, weeds, molluscs, etc., that destroy the plant crop and spread diseases. Aldrin and dieldrin are the names of some common pesticides.
Herbicides are pesticides specially meant for killing weeds. For example, sodium chlorate (NaClO3), sodium arsenite (Na3AsO3) etc.
Question 16.
What do you mean by green chemistry? How will it help in reducing environmental pollution?
Answer:
Green chemistry is a production process that aims at using the existing knowledge and principles of chemistry for developing and implementing chemical products and processes to reduce the use and generation of substances hazardous to the environment.
The release of different harmful chemicals (particulates, gases, organic and inorganic wastes) causes environmental pollution. In green chemistry, the reactants to be used in chemical reactions are chosen in such a way that the yield of the end products is up to 100%. This prevents or limits chemical pollutants from being introduced into the environment. Through the efforts of green chemists, H2O2 has replaced tetrachloroethane and chlorine gas in drying and bleaching of paper.
Question 17.
What would have happened if the greenhouse gases were totally missing in the earth’s atmosphere? Discuss.
Answer:
Earth’s most abundant greenhouse gases are CO2, CH4, O3 , CFCs and water vapour. These gases are present near the Earth’s surface. They absorb solar energy that is radiated back from the surface of the Earth. The absorption of radiation results in the heating up of the atmosphere. Hence, greenhouse gases are essential for maintaining the temperature of the Earth for the sustenance of life.
In the absence of greenhouse gases, the average temperature of the Earth will decrease drastically, making it uninhabitable. As a result, life on Earth would be impossible.
Question 18.
A large number of fish are suddenly floating dead on a lake. There is no evidence of toxic dumping but you find an abundance of phytoplankton. Suggest a reason for the fish kill.
Answer:
The amount of dissolved oxygen present in water is limited. The abundance of phytoplanktons causes depletion of this dissolved oxygen. This is because phytoplanktons are degraded by bacteria present in water. For their decomposition they require a large amount of oxygen. Hence, they consume the oxygen dissolved in water. As a result, the BOD level of water drops below 6 ppm, inhibiting the growth of fish and causing excessive fish kill.
Question 19.
How can domestic waste be used as manure?
Answer:
Depending upon the nature of the waste, domestic waste can be segregated into two categories i.e., biodegradable and non-biodegradable. Biodegradable waste such as leaves, rotten food, etc. should be deposited in land fills, where they get decomposed aerobically and anaerobically into manure. Non-biodegradable waste (which cannot be degraded) such as plastic, glass, metal scraps etc. should be sent for recycling.
![]()
Question 20.
For your agricultural field or garden you have developed a compost producing pit. Discuss the process in the light of bad ’ odour, flies and recycling of wastes for a good produce.
Answer:
It is essential to take proper care of the compost producing pit in order to protect ourselves from bad odour and flies.
It should be kept covered to minimize bad odour and prevent flies from entering it.
The recyclable waste should not be dumped in the compost producing pit. It should be sent to the industries through vendors for recycling.
