Punjab State Board PSEB 12th Class Chemistry Book Solutions Chapter 6 General Principles and Processes of Isolation of Elements Textbook Exercise Questions and Answers.
PSEB 12th Class Chemistry Important Questions Chapter 6 General Principles and Processes of Isolation of Elements
Very Short Answer Type Questions
Question 1.
Zinc acts as a reducing agent in the extraction of silver. Comment.
Answer:
Zinc acts as a reducing agent in the extraction of silver. It reduces Ag+ to Ag and itself get oxidised to Zn2+.
2Na[Ag(CN)2] + Zn → Na2[Zn(CN)4] + 2Ag↓
Question 2.
Winch reducing agent is employed to get copper from the leached low grade copper ore?
Answer:
Scrap iron, Cu2+(aq) + Fe(s) → Cu(s) + Fe2+(aq)
or H2 gas, Cu2+(aq) + H2(g) → Cu(s) + 2H+(aq)
Question 3.
Name the method used for refining of zirconium.
Answer:
Van Arkel method
![]()
Question 4.
Name the method that is used for refining of nickel.
Answer:
Mond process (Vapour phase refining)
Question 5.
Name the method used for refining of copper metal.
Answer:
Electrolytic refining
Question 6.
Although carbon and hydrogen are better reducing agents but they are not used to reduce metallic oxides at high temperatures. Why?
Answer:
At high temperature carbon and hydrogen react with metals to form carbides and hydrides respectively.
![]()
Question 7.
What is the function of collectors in the froth floatation process for the concentration of ores?
Answer:
Collectors (e.g., pine oil, xanthates etc.) enhance non-wettability of the ore particles.
Question 8.
Why is it that only sulphide ores are concentrated by froth floatation process?
Answer:
This is because the sulphide ore particles are preferentially wetted by oil and gangue particles are preferentially wetted by water.
Question 9.
At temperatures above 1073 K, coke can be used to reduce FeO to Fe. How can you justify this reduction with Ellingham diagram?
Answer:
Using Ellingham diagram, we observe that at temperature greater than 1073 K; △G(C, CO) < △G (Fe, FeO).
Hence, coke can reduce FeO to Fe.
![]()
Question 10.
The mixture of compounds A and B is passed through a column of Al2O3 by using alcohol as eluant. Compound A is eluted in preference to compound B. Which of the compounds A or B, is more readily adsorbed on the column?
Answer:
Since, compound ‘A’ comes out before compound ‘B’ the compound ‘B’ is more readily adsorbed on the column.
Short Answer Type Questions
Question 1.
Write the role of:
(i) I2 in the van Arkel method of refining.
(ii) Dilute NaCN in the extraction of silver.
Answer:
(i) Impure titanium is heated with iodine to form volatile TiI4, which decomposes on tungsten filament at high temperature to give pure titanium.

(ii) Dilute NaCN forms a soluble complex with Ag or Ag2S while the impurities remain unaffected which are filtered off.
4Ag + 8NaCN + O2 + 2H2O → 4Na[Ag(CN)2] + 4NaOH
or
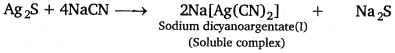
![]()
Question 2.
Describe the role of
(i) Iodine in the refining of zirconium.
(ii) NaCN in the extraction of gold from gold ore.
Write chemical equations for the involved reactions.
Answer:
(i) Impure zirconium is heated with iodine to form volatile compound ZrI4 which on further heating over tungsten filament decomposes to give pure zirconium.
![]()
(ii) Gold ore is leached with dilute solution of NaCN in the presence of air from which the metal is obtained later by replacement.
4Au + 8NaCN + O2 + 2H2O → 4Na[Au(CN)2] + 4NaOH
Question 3.
Explain the role of each of the following in the extraction of metals from their ores:
(i) CO in the extraction of nickel.
(ii) Zinc in the extraction of silver.
Answer:
(i) CO in the extraction of nickel: Impure nickel is heated in a stream of carbon monoxide when volatile nickel tetracarbonyl is formed and the impurities are left behind in the solid state. The vapour of nickel tetracarbonyl is taken to a decomposer chamber maintained at 450-470 K where it decomposes to give pure nickel metal and carbon monoxide.

(ii) Zinc in the extraction of silver : Silver present in the ore is leached with dilute solution of NaCN in the presence of air or oxygen to form a soluble complex.
![]()
Silver is then recovered from the complex by displacement method using more electropositive zinc metal.
2[Ag(CN)2]– (aq) + Zn(s) → 2Ag(s) + [Zn(CN)2]2- (aq)
![]()
Question 4.
Wrought iron is the purest form of iron. Write a reaction used for the preparation of wrought iron from cast iron. How can the impurities of sulphur, silicon and phosphorus be removed from cast iron?
Answer:

This reaction takes place in reverberatory furnace lined with haematite.
(b) Limestone is added as flux. Impurities of S, Si and P oxidise and pass into slag. The metal is removed and freed from slag by passing through rollers.
Question 5.
Write the chemical reactions involved in the extraction of gold by cyanide process. Also give the role of zinc in the reaction.
Answer:
(i) 4Au(s) + 8CN– (aq) + 2H2O(aq) + O2(g) → 4[Au(CN)2]– (aq) + 4OH–(aq)
(ii) 2[Au(CN)2]– (aq) + Zn(s) → 2Au(s) + [Zn(CN)4]2- (aq)
Zinc acts as a reducing agent in this reaction.
![]()
Question 6.
Describe the role of
(i) NaCN in the extraction of gold from its ore.
(ii) Cryolite in the extraction of aluminium from pure alumina.
(iii) CO in the purification of nickel.
Answer:
(i) Gold is leached with a dilute solution of NaCN in the presence of air.
(ii) Cryolite lowers the high melting point of alumina and makes it a good conductor of electricity.
(iii) CO forms a volatile complex with metal nickel which is further decomposed to give pure Ni metal.
Long Answer Type Questions
Question 1.
(a) Explain how an element can be extracted using an oxidation reaction?
(b) What do you mean by refining? Mention some of the methods used for refining of metals.
Answer:
(a) Some of the extractions, particularly of non-metals are based upon oxidation.
A very common example of extraction based on oxidation is the extraction of chlorine from brine (Chlorine is abundant in sea water as common salt).
2Cl–(aq) + 2H2O(l) → 2OH–(aq) + H2(g) + Cl2(g)
The △G0 for this reaction is + 422 kJ. When it is converted to E0 (using △G0 = -nE0F), we get E0 = -2.2 V. Naturally, it will require an external e.m.f. that is greater than 2.2 V. But the electrolysis requires an excess potential to overcome some other hindering reactions. Thus, Cl2 is obtained by electrolysis giving out H2 and aqueous NaOH as by products. Electrolysis of molten NaCl is also carried out. But in that case, Na metal is produced and not NaOH.
The extraction of gold and silver involves leaching the metal with CN–. This is also an oxidation reaction (Ag → Ag+ or Au → Au+). The metal is later recovered by displacement method.
4Au(s) + 8CN–(aq) + 2H2O(aq) + O2(g) → 4[Au(CN2)]–(aq) + 4OH(aq)
2[Au(CN)2]–(aq) + Zn(s) → 2Au(s) + [Zn(CN)4]2- (aq)
In this reaction zinc acts as a reducing agent.
(b) A metal extracted by any method is usually contaiminated with some impurity. For obtaining metals of high purity, several techniques are used depending upon the difference in properties of the metal and the impurity. The process is called refining. Some of them are listed below :
- Distillation,
- Liquation,
- Electrolysis,
- Zone-refining,
- Vapour phase refining,
- Chromatographic methods.
![]()
Question 2.
How is the concept of coupling reactions useful in explaining the occurrence of non-spontaneous thermochemical reactions? Explain giving an example?
Answer:
Coupled reactions : Many reactions which are non-spontaneous (△G⊖ is positive) can be made to occur spontaneously if these are coupled with reactions having larger negative free energy. By coupling means carrying out simultaneously both non- spontaneous and spontaneous reactions. For example, decomposition of Fe2O3into iron is a non-spontaneous reaction (△G⊖ = +1487 kJ mol-1). However, this decomposition can take place spontaneously if carbon monoxide is simultaneously burnt in oxygen (△G⊖ = – 514.4 kJ mol-1).
2Fe2O3(s) → 4Fe(s) + 3O2(g); …(i);
△G⊖ = + 1487.0 kJmol-1
2CO(g) + O2(g) → 2CO2(g); … (ii);
△G⊖ = -514.4 kJmol-1
Multiplying equation (ii) by 3 and then adding to equation (i), we get
6CO(g) + 3O2(g) → 6CO2(g) △G⊖ = -1543.2 kJ mol-1
2Fe2O3 (s) → 4Fe(s) + 3O2(s) △G⊖ = +1487.0 kJ mol-1
2Fe2O3(s) + 6CO(g) → 4Fe(s) + 6CO2(g) △G⊖ = – 56.2 kJ mol-1
Since, △G⊖ in the reduction of Fe2O3 with CO is negative, therefore, the reaction is feasible and spontaneous.
