Punjab State Board PSEB 12th Class Chemistry Book Solutions Chapter 6 General Principles and Processes of Isolation of Elements Textbook Exercise Questions and Answers.
PSEB Solutions for Class 12 Chemistry Chapter 6 General Principles and Processes of Isolation of Elements
PSEB 12th Class Chemistry Guide General Principles and Processes of Isolation of Elements InText Questions and Answers
Question 1.
Copper can be extracted by hydrometallurgy but not zinc% Explain.
Answer:
The E⊖ value of zinc (Zn2+/Zn = – 0.76 V) is lower than that of copper (Cu2+/Cu = 0.34 V). This means that .zinc is a stronger reducing agent and can displace copper from solution of Cu2+ ions.
Zn(s) + Cu2+ (aq) → Zn2+(aq) + Cu(s)
In order to extract zinc by hydrometallurgy, we need stronger reducing agent like
![]()
![]() etc. However, all these metals reduce water to hydrogen gas. Therefore, these metals cannot be used to displace Zn from solution of Zn2+ ions. Thus, copper can be extracted by hydrometallurgy but not zinc.
etc. However, all these metals reduce water to hydrogen gas. Therefore, these metals cannot be used to displace Zn from solution of Zn2+ ions. Thus, copper can be extracted by hydrometallurgy but not zinc.
Question 2.
What is the role of depressant in froth floatation process?
Answer:
In the froth floatation process, the role of the depressants is to separate two sulphide ores by selectively preventing one ore from forming froth. For example, to separate two sulphide ores (ZnS and PbS), NaCN is used as a depressant which selectively allows PbS to come with froth, but prevents ZnS from coming to froth. This happens because NaCN reacts with ZnS to form Na2[Zn(CN)4].
4NaCN + ZnS → Na2[Zn(CN)4] + Na2S
Question 3.
Why is the extraction of copper from pyrites more difficult than that from its oxide ore through reduction?
Answer:
The Gibbs free energy of formation (△fG) of Cu2S is less than that of H2S and CS2. Therefore, H2 and C cannot reduce Cu2S to Cu.
On the other hand, the Gibbs free energy of formation of Cu2O is greater than that of CO. Hence, C can reduce Cu2O to Cu.
C(s) + Cu2O(s) → 2Cu(s) + CO(g)
Hence, the extraction of copper from its pyrite ore is difficult than from its oxide ore through reduction.
![]()
Question 4.
Explain:
(i) Zone refining
(ii) Column chromatography.
Answer:
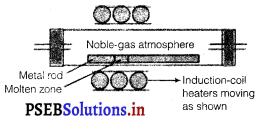
(i) Zone refining : This method is based on the principle that impurities are more soluble in the molten state of metal (the melt) than in the solid state. In the process of zone refining, a circular mobile heater is fixed at one end of a rod of impure metal. As the heater moves, the molten zone of the rod also moves with it. As a result, pure metal crystallizes out of the melt and the impurities pass onto the adjacent molten zone. This process is repeated several times, which leads to the segregation of impurities at one end of the rod. Then, the end with the impurities is cut off. Silicon, boron, gallium, indium etc. can be purified by this process.
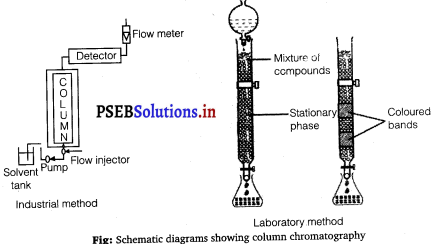
(ii) Column chromatography : Column chromatography is a technique used to separate different components of a mixture. It is a very useful technique used for the purification of elements available in minute quantities. It is also used to remove the impurities that are not very different in chemical properties from the element to be purified. Chromatography is based on the principle that different components of a mixture are differently adsorbed on an adsorbent. In chromatography, there are two phases: mobile phase and stationary phase. The stationary phase is immobile and immiscible. Al2O3 column is usually used as the stationary phase in column chromatography. The mobile phase may be a gas, liquid, or supercritical fluid in which the sample extract is dissolved. Then, the mobile phase is forced to move through the stationary phase. The component that is more strongly adsorbed on the column takes a longer time to travel through it than the component that is weakly adsorbed. The adsorbed components are then removed (eluted) using a suitable solvent (eluant).
Question 5.
Out of C and CO, which is a better reducing agent at 673 K?
Answer:
At 673 K, the value of △G(CO,CO2) is less than that of △G(C,CO).
Therefore, CO can be oxidised more easily to CO2 than C to CO. Hence, CO is a better reducing agent than C at 673 K.
Question 6.
Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present ?
Answer:
In electrolytic refining of copper, the common elements present in anode mud are selenium, tellurium, silver, gold, platinum, and antimony.
These elements are very less reactive and are not affected during the purification process. Hence, they settle down below the anode as anode mud.
![]()
Question 7.
Write down the reactions taking place in different zones in the blast furnace during the extraction of iron.
Answer:
Reactions in blast furnace are as follows :
(a) Reactions at lower temperature range (500 to 800 K)
3Fe2O3 + CO → 2Fe3O4 + CO2
Fe3O4 + 4CO → 3Fe + 4CO2
Fe2O3 + C0 → 2FeO + CO2
(b) Reactions at higher temperature range (900-1500 K)
C + CO2 → 2CO
FeO + CO → Fe + CO2
Question 8.
Write chemical reactions taking place in the extraction of zinc from zinc blende. .
Answer:
The different steps involved in the extraction of zinc from zinc blende (ZnS) are given below:
(i) Concentration of ore : First, the gangue from zinc blende is removed by the froth floatation method.
(ii) Conversion to oxide (Roasting) : Sulphide ore is converted into oxide by the process of roasting. In this process, ZnS is heated in a regular supply of air in a furnace at a temperature, which is below the melting point of Zn.
2ZnS + 3O22 → 2ZnO + 2SO2
(iii) Extraction of zinc from zinc oxide (Reduction) : Zinc is extracted from zinc oxide by the process of reduction. The reduction of zinc oxide is carried out by mixing it with powdered coke and then, heating it at 1673 K.
![]()
(iv) Electrolytic refining: Zinc can be refined by the process of electrolytic refining. In this process, impure zinc is made the anode while a pure copper strip is made the cathode. The electrolyte used is an acidified solution of zinc sulphate (ZnSO4). Electrolysis results in the transfer of zinc in pure form from the anode to the cathode.
Anode : Zn → Zn2+ + 2e–
Cathode : Zn2+ + 2e– → Zn
Question 9.
State the role of silica in the metallurgy of copper.
Answer:
During the roasting of pyrite ore, a mixture of FeO and Cu2O is obtained.

The role of silica in the metallurgy of copper is to remove the iron oxide obtained during the process of rosting as ‘slag’. If the sulphide ore of copper contains iron, then silica (SiO2) is added as flux before roasting. Then, FeO combines with silica to form iron silicate, FeSiO3 (slag).
![]()
![]()
Question 10.
What is meant by the term “chromatography”?
Answer:
Chromatography is a collective term used for a family of laboratory techniques for the separation of mixtures. The term is derived from Greek words ‘chroma’ meaning ‘colour’ and ‘graphy5 meaning ‘writing’. Chromatographic techniques are based on the principle that different components are absorbed differently on an absorbent. There are several chromatographic techniques such as paper chromatography, column chromatography, gas chromatography, etc.
Question 11.
What criterion is followed for the selection of the stationary phase in chromatography?
Answer:
The stationary phase is selected in such a way that the components of the sample have different solubility’s in the phase. Hence, different components have different rates of movement through the stationary phase and as a result, can be separated from each other.
Question 12.
Describe a method for refining nickel.
Answer:
Nickel is refined by Mond’s process. In this process, nickel is heated in the presence of carbon monoxide to form nickel tetracarbonyl, which is a volatile complex.
![]()
Then, the obtained nickel tetracarbonyl is decomposed by subjecting it to a higher temperature (450-470 K) to obtain pure nickel metal.

![]()
Question 13.
How can you separate alumina from silica in a bauxite ore associated with silica? Give equations, if any.
Answer:
(i) To separate alumina from silica in a bauxite ore associated with silica, first the powdered ore is digested with a concentrated NaOH solution at 473-523 K and 35-36 bar pressure. This results in the leaching out of alumina (Al2O3) as sodium aluminate and silica (SiO2) as sodium silicate leaving the impurities behind.
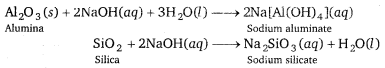
(ii) Then, CO2gas is passed through the resulting solution to neutralise the aluminate in the solution, which results in the precipitation of hydrated alumina. To induce precipitation, the solution is seeded with freshly prepared samples of hydrated alumina.
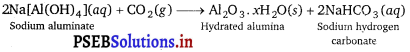
(iii) During this process, sodium silicate remains in the solution. The obtained hydrated alumina is filtered, dried, and heated to get back pure alumina.
![]()
Question 14.
Giving examples, differentiate between ‘roasting’ and ‘calcination’.
Answer:
| Roasting | Calcination |
| 1. Sulphur dioxide is produced along with metal oxide. | Carbon dioxide is produced along with metal oxide. |
| 2. Ore is heated in the presence of excess of air or oxygen. | Ore is heated in the absence or limited supply of air or O2. |
| 3. Volatile impurities are removed as oxides, such as SO2, As2O3, etc. |
Water and organic impurities are removed. |
Question 15.
How is ‘cast iron’ different from ‘pig iron”?
Answer:
The iron obtained from blast furnaces is known as pig iron. It contains around 4% carbon and many impurities such as S, P, Si, Mn in smaller amounts.
Cast iron is obtained by melting pig iron and coke using a hot air blast. It contains a lower amount of carbon (3%) than pig iron. Unlike pig iron, cast iron is extremely hard and brittle.
![]()
Question 16.
Differentiate between “minerals” and “ores”.
Answer:
| Mineral | Ore |
| Naturally occurring substances of metals present in the earth’s crust are called minerals. | Minerals which can be used to obtain the metal profitably are cahed ores. |
| All minerals are not ores. | All ores are essentially minerals too. |
| e.g,, bauxite (Al2O3-xH2O) and clay (A12O3 -2SiO2 -2H2O) | e.g., bauxite (A12O3 ∙xH2O) |
Question 17.
Why copper matte is put in silica lined converter?
Answer:
Copper matte contains Cu2S and FeS. Copper matte is put in a silica-lined converter to remove the remaining FeO and FeS present in the matte as slag (FeSiO3). Also, some silica is added to the silica-lined converter. Then, a hot air blast is blown. As a result, the remaining FeS and FeO are converted to iron silicate (FeSiO3) and Cu2S is converted into metallic copper.
2FeS + 3O2 → 2FeO + 2SO2
FeO + SiO2 → FeSiO2
2Cu2S + 3O2 → 2Cu2O + 2SO2
2Cu2O + Cu2S → 6Cu + SO2
Question 18.
What is the role of cryolite in the metallurgy of aluminium?
Answer:
Cryolite (Na3AlF6) has two roles in the metallurgy of aluminium :
- To decrease the melting point of the mixture from 2323 K to 1140 K.
- To increase the electrical conductivity of Al2O3.
![]()
Question 19.
How is leaching carried out in case of low grade copper ores?
Answer:
In case of low grade copper ores, leaching is carried out using acid or bacteria in the presence of air. In this process, copper goes into the solution as Cu2+ ions.
Cu(s) + 2H+(aq) + \(\frac{1}{2}\)O2(g) → Cu2+(aq) + 2H2O(l)
The resulting solution is treated with scrap iron or H2 to get metallic copper.
Cu2+(aq) + H2(g) → Cu(s) + 2H+(aq)
Question 20.
Why is zinc not extracted from zinc oxide through reduction using CO?
Answer:
The standard free energy of formation (△fG⊖) of CO2 from CO is
higher than that of the formation of ZnO from Zn. Therefore, CO cannot be used to reduce ZnO to Zn.

Question 21.
The value of △fG⊖ for formation of Cr2O3 is – 540 kJmol-1 and that of A2O3 is – 827 kJ mol-1. Is the reduction of Cr2O3 possible with Al?
Answer:
The two thermochemial equations may be written as
(i) 2Al + \(\frac{1}{2}\)O2 → Al2O3 △fG⊖ = -827kJmol-1
(ii) 2Cr + \(\frac{1}{2}\)O2 → Cr2O3 △fG⊖ = -540kJmol-1
Subtracting equation (ii) from (i), we have
2Al + Cr2O3 → Al2O3 + 2Cr
△fG⊖ = -827-(-540)
= -287kJmol-1
As △fG⊖ for the reduction reaction of Cr2O3 by Al is negative, this reaction is possible.
![]()
Question 22.
Out of C and CO, which is a better reducing agent for ZnO?
Answer:
The free energy of formation (△fG⊖) of CO from C becomes lower at temperatures above 1120 K whereas that of CO2 from C becomes lower above 1323 K than △fG⊖ of ZnO. However, △fG⊖ of CO2 from CO is always higher than that of ZnO. Therefore, C and reduce ZnO to Zn but not CO. Therefore, out of C can CO, C is a better reducing agent than CO for ZnO.
Question 23.
The choice of a reducing agent in a particular case depends on thermodynamic factor. How far do you agree with this statement? Support your opinion with two examples.
Answer:
For any spontaneous reaction, the Gibbs free energy change (△G) must be negative. △G = △H – T△S where △H is the enthalpy change during the reaction, T is the absolute temperature and △S is the change in entropy.
Consider the Ellingham diagram (given below) for some metal oxides. From the diagram, it is evident that metals for which the free energy of formation of their oxides is more negative can reduce those metal oxides for which the free energy of formation of their respective oxides is less negative. In other words, any metal will reduce the oxide of other metals which lie above it in the Ellingham diagram because the free energy will become more negative by an amount equal to the difference in the two graphs at that particular temperature. Thus, Al reduces FeO, Cr2O3 and NiO in Thermite reaction, but Al will not reduce MgO at a temperature below 1773 K.
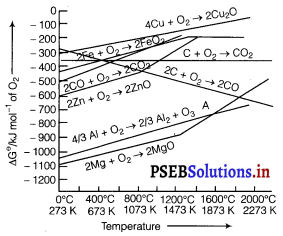
It can be followed that:
(i) 2Al + Cr2O3→ Al2O3 + 2Cr
(Aluminothermic process)
(ii) 2Al + Fe2O3 → Al2O3 + 2Fe are spontaneous.
But Al can’t be used to reduce MgO below 1500°C. From the above it is clear that thermodynamic considerations help us in choosing a suitable reducing agent in metallurgy.
![]()
Question 24.
Name the processes from which chlorine is obtained as a by-product. What will happen if an aqueous solution of NaCl is subjected to electrolysis?
Answer:
(i) Down’s process for the manufacture of Na metal: When molten NaCl is subjected to electrolysis, chlorine is obtained as a by product at anode because in molten state only Na+ and Cl– ions are present.
NaCl (melt) → Na+ (melt) + Cl– (melt)
At cathode : Na+ (melt) + e– → Na(s)
At anode : Cl–(melt) → Cl(g) + e–
(ii) Manufacture of NaOH : If an aqueous solution of NaCl is electrolysed, Cl2 will be obtained at the anode but at the cathode, H2 will be obtained instead of Na. This is because the standard reduction potential of Na (E⊖ = – 2.71 V) is more negative than that of H2O (E⊖ = – 0.83 V). Hence, H2O will get preference to get reduced at the cathode and as a result, H2 is evolved.
NaCl(aq) → Na+(aq) + Cl– (aq)
H2O ⇌ H+(aq) + OH–(aq)
At cathode : 2H2O(l) + 2e– → H2(g) + 2OH– (aq)
At anode : Cl– (melt) → Cl(g) + e–
2Cl (g) → Cl2(g)
H2 gas is obtained at cathode; chlorine gas at anode and NaOH is formed in the solution.
Na+(aq) + OH–(aq) → NaOH (aq)
Question 25.
What is the role of graphite rod in the electrometallurgy of aluminium?
Answer:
In the electrometallurgy of aluminium, a fused mixture of purified alumina (Al2O3), cryolite (Na3AlF6) and fluorspar (CaF2) is electrolysed. In this electrolysis, graphite is used as the anode and graphite-lined iron is used as the cathode. During the electrolysis, A1 is liberated at the cathode, while CO and CO2 are liberated at the anode, according to the following equation :
At cathode: Al3+(melt) + 3e– → Al(l)
At anode: C(s) + O2- (melt) → CO(g) + 2e–
C(s) + 2O2- (melt) → CO2(g) + 4e–
If a metal is used instead of graphite as the anode, then 02will be liberated. This will not only oxidise the metal of the electrode, but also convert some of the A1 liberated at the cathode back into Al2O3. Hence, graphite is used for preventing the formation of O2 at the anode.
![]()
Question 26.
Outline the principles of refining of metals by the following methods:
(i) Zone refining
(ii) Electrolytic refining
(iii) Vapour phase refining
Answer:
(i) Zone refining : This method is based on the principle that impurities are more soluble in the molten state of metal (the melt) than in the solid state. In the process of zone refining, a circular mobile heater is fixed at one end of a rod of impure metal. As the heater moves, the molten zone of the rod also moves with it. As a result, pure metal crystallizes out of the melt and the impurities pass onto the adjacent molten zone. This process is repeated several times, which leads to the segregation of impurities at one end of the rod. Then, the end with the impurities is cut off. Silicon, boron, gallium, indium etc. can be purified by this process.

(ii) Electrolytic refining : Electrolytic refining is the process of refining impure metals by using electricity. In this process, impure metal is made the anode and a strip of pure metal is made the cathode. A solution of a soluble salt of the same metal is taken as the electrolyte. When an electric current is passed, metal ions from the electrolyte are deposited at the cathode as pure metal and the impure metal from the anode dissolves into the electrolyte in the form of ions. The impurities present in the impure metal gets collected below the anode. This is known as anode mud.
At anode: M → Mn+ + ne–
At cathode: Mn+ + ne– → M
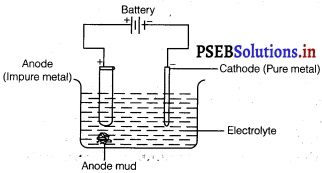
(iii) Vapour phase refining : Vapour phase refining is the process of refining metal by converting it into its volatile compound and then, decomposing it to obtain a pure metal. To carry out this process,
- the metal should form a volatile compound with an available reagent, and
- the volatile compound should be easily decomposable so that the metal can be easily recovered.
Nickel, zirconium, and titanium are refined using this method.
Question 27.
Predict conditions under which Al might be expected to reduce MgO. (Hint: See Intext Question 4)
Answer:
The equations for the formation of two oxides are :
\(\frac{4}{3}\)Al(s) + O2(g) → \(\frac{2}{3}\)Al2O3(s)
2Mg(s) + O2(g) → 2MgO(s)
If we observe the plots for the formation of the two oxides on the Ellingham diagram, we find the two curves intersect each other at a certain point. The corresponding value of △fG⊖ becomes zero for the reduction of MgO by aluminium metal.
2MgO(s) + \(\frac{4}{3}\)Al(s) ⇌ 2Mg(s) + \(\frac{2}{3}\)Al2O3(s)
This means that the reduction of MgO by A1 metal cannot occur below this temperature (1665 K). Instead, Mg can reduce Al2O3 to Al below 1665 K.
Aluminium metal (Al) can reduce MgO to Mg above 1665 K
because △fG⊖ for Al2O3 is less as compared to that of MgO.
![]()
![]()
Chemistry Guide for Class 12 PSEB General Principles and Processes of Isolation of Elements Textbook Questions and Answers
Question 1.
Which of the ores mentioned in Table 6.1 can be concentrated by magnetic separation method?
Answer:
If the ore or the gangue can be attracted by the magnetic field, then the ore can be concentrated by the process of magnetic separation. The ores of iron such as haematite (Fe2O3), magnetite (Fe3O4), siderite (FeCO3) and iron pyrites (FeS2) can be separated by the process of magnetic separation.
Question 2.
What is the significance of leaching in the extraction of aluminium?
Answer:
In the extraction of aluminium, the significance of leaching is to concentrate pure alumina (Al2O3) from bauxite ore. Bauxite usually contains silica, iron oxide, and titanium oxide as impurities. In the process of leaching, alumina is concentrated by digesting the powdered ore with a concentrated solution of NaOH at 473-523 K and 35-36 bar. Under these conditions, alumina (Al2O3) dissolves as sodium meta-aluminate and silica (SiO2) dissolves as sodium silicate leaving the impurities behind.
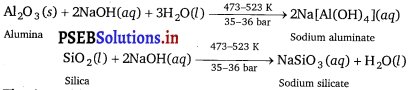
The impurities are then filtered and the solution is neutralised by passing CO2 gas. In this process, hydrated Al2O3 gets precipitated and sodium silicate remains in the solution. Precipitation is induced by seeding the solution with freshly prepared samples of hydrated Al2O3.
2Na[Al(OH)4](aq) + CO2(g) → Al2O3∙xH2O(S) + 2NaHCO3(aq)
Hydrated alumina
Hydrated alumina Al2O3∙xH2O is filtered, dried, and heated to give back pure alumina (Al2O3).
![]()
![]()
Question 3.
The reaction,
Cr2O3 + 2Al > Al2O3 + 2Cr (△fG⊖ = -421kJ) is thermodynamically feasible as is apparent from the Gibbs energy value.
Why does it not take place at room temperature?
Answer:
The change in Gibbs energy is related to the equilibrium constant, K as
△G = – RT in K
At room temperature, all reactants and products of the given reaction are in the solid state. As a result, equilibrium does not exist between the reactants and th e prod ac ts lienee, the reaction does not take place at room temperature.
However, at a higher temperature, chromium melts and the reaction takes place.
We also know that according to the equation
△G = △H – T△S,
Increasing the temperature increases die value of T△S, making the value of △G more and more negative. Therefore, the reaction becomes more and more feasible as the temperature is increased.
Question 4.
Is it true that under certain conditions. Mg can reduce Al2O3 and Al can reduce MgO? What are those conditions?
Answer:
If we look at the Ellingbam diagram wo observe that the plots for Al and Mg cross each other at 1350°C (1623k) Below this temperature Mg can reduce Al2O3 and above this temperature., Al can reduce MgO.
