Punjab State Board PSEB 12th Class Chemistry Book Solutions Chapter 12 Aldehydes, Ketones, and Carboxylic Acids Textbook Exercise Questions and Answers.
PSEB Solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones, and Carboxylic Acids
PSEB 12th Class Chemistry Guide Aldehydes, Ketones, and Carboxylic Acids InText Questions and Answers
Question 1.
What is meant by the following terms? Give an example of the reaction in each case.
(i) Cyanohydrin
(ii) Acetal
(iii) Semicarbazone
(iv) Aldol
(v) Hemiaeetal
(vi) Oxime
(vii) Ketal
(viii) Imine
(ix) 2, 4-DNP derivative
(x) Schiffs base.
Answer:
(i) Aldehydes and ketones react with hydrogen cyanide (HCN) to yield cyanohydrins.
It is catalyzed by a base and the generated cyanide ion (CN–) being a stronger nucleophile readily adds to carbonyl compounds to yield corresponding cyanohydrin.
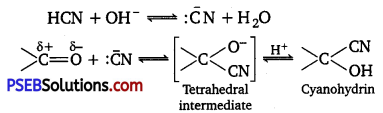
(ii) gem-Dialkoxy compounds in which the two alkoxy groups are present on the terminal carbon atom are called acetals. These are produced by the action of an aldehyde with two equivalents of a monohydric alcohol in the presence of dry HCl gas.
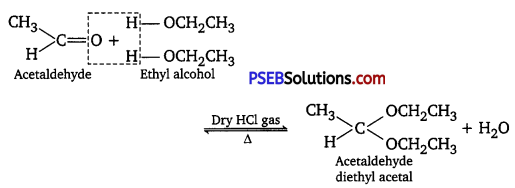
These are easily hydrolyzed by dilute mineral acids to regenerate the original aldehydes. Therefore, these are used for the protection of aldehydic group in organic synthesis.
(iii) Semicarbazones are derivatives of aldehydes and ketones and are produced by the action of semicarbazide on them in weak acidic medium.
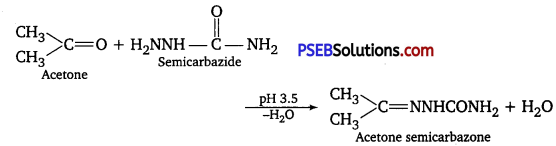
These are used for identification and characterization of aldehydes and ketones.
(iv) Aldehydes and ketones having at least one α -hydrogen undergo a reaction in the presence of dilute alkali as catalyst to form f3-hydroxy aldehydes (aldol) or β-hydroxy ketones (ketol), respectively. This is known as aldol condensation.
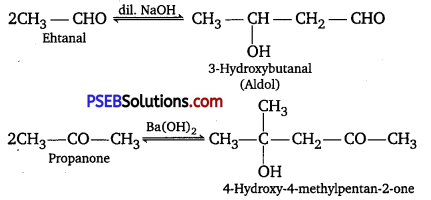
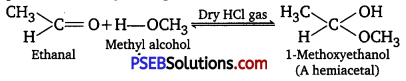
(v) gem-Alkoxyalcohols are called hemiacetals. These are produced by addition of one molecule of a monohydric alcohol to an aldehyde in presence of dry HCl gas.
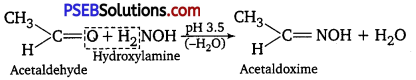
(vi) Oximes are produced when aldehydes or ketones react with hydroxylamine in weak acidic medium.
(vii) gem-Dialkoxyalkanes are called ketals. In ketals, the two alkoxy groups are present on the same carbon within the chain. These are produced when a ketone is heated with ethylene glycol in presence of diy HCl gas or p-toluene sulphonic acid (PTS).

These are easily hydrolyzed by dilute mineral acids to regenerate the original ketones. Therefore, ketals are used for protection of keto groups in organic synthesis.
(viii) Compounds containing ![]() group are called imines. These are produced when aldehydes and ketones react with ammonia derivatives.
group are called imines. These are produced when aldehydes and ketones react with ammonia derivatives.
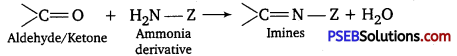
Z = Alkyl, aryl – NH2,-OH,-C6H5NH,-NHCONH2 etc.
(ix) 2, 4-Dinitrophenythydrazones (i.e., 2, 4-DNP derivatives) are produced when aldehydes or ketones react with 2, 4-dinitrophenyl hydrazine in weakly acidic medium.
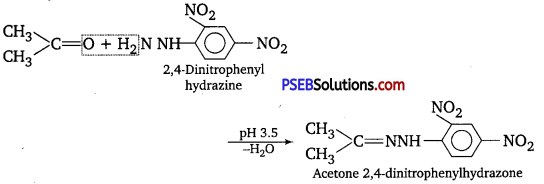
2, 4-DNP derivatives are used for identification and characterization of aldehydes and ketones.
(x) Aldehydes and ketones react with primary aliphatic or aromatic amines to form azomethines or Schiffs bases.
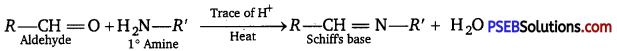
Question 2.
Name the following compounds according to IUPAC system of nomenclature:
(i) CH3CH(CH3)CH2CH2CHO
(ii) CH3CH2COCH(C2H5)CH2CH2Cl
(iii) CH3CH=CHCHO
(iv) CH3COCH2COCH3
(v) CH3CH(CH3)CH2C(CH3)2COCH3
(vi) (CH3)3CCH2COOH
(vii) OHCC6H4CHO-p
Answer:
(i) 4-Methylpentanal
(ii) 6-Chloro-4-ethylhexan-3-one
(iii) But-2-en-l-al
(iv) Pentane-2,4-dione
(v) 3,3,5-Trimethylhexan-2-one
(vi) 3,3-Dimethylbutanoic acid
(vii) Benzene-1,4-dicarbaldehyde
![]()
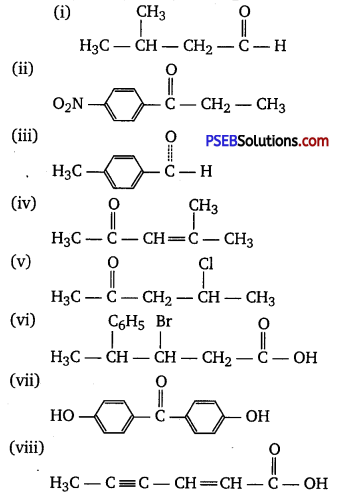
Question 3.
Draw the structures of the following compounds.
(i) 3-Methylbutanal
(ii) p-Nitropropiophenone
(iii) p-Methylbenz aldehyde
(iv) 4-Methylpent-3-en-2-one
(v) 4-Chloropentan-2-one
(vi) 3-Bromo-4-phenylpentanoic acid
(vii) p,p’-Dihydroxybenzophenone
(viii) Hex-2-en-4-ynoic acid
Answer:
Question 4.
Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
(i) CH3CO(CH2)4CH3
(ii) CH3CH2CH Br CH2CH(CH3) CHO
(iii) CH3(CH2)5CHO
(vi) PhCOPh
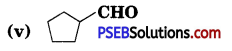
Answer:
| IUPAC Name | Common Name |
| (i)Heptane-2-one | Methyl n-propyl ketone |
| (ii) 4-Bromo-2-methyl hexanal | γ -Bromo- α –methyl caproaldehyde |
| (iii) Heptanal | n-heptyl aldehyde |
| (iv) 3-Phenyprop-2-enal | Β- Phenylacrole in |
| (v) Cyclopentane-carbaldehyde | Cyclopentane carbaldehyde |
| (vi) Diphenyl methanone | Benzophenone |
Question 5.
Draw structures of the following derivatives.
(i) The 2,4-dinitrophenylhydrazone of benzaldehyde
(ii) Cyclopropanone oxime
(iii) Acetaldehydedimethylacetal
(iv) The semicarbazone of cyclobutanone
(v) The ethylene ketal of hexan-3-one
(vi) The methyl hemiacetal of formaldehyde
Answer:
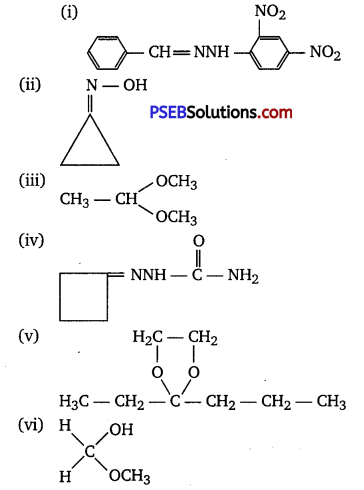
Question 6.
Predict the products formed when cyclohexane carbaldehyde reacts with following reagents.
(i) PhMgBr and then H3O+
(ii) Tollens’ reagent
(iii) Semicarbazide and weak acid
(iv) Excess ethanol and acid
(v) Zinc amalgam and dilute hydrochloric acid
Answer:
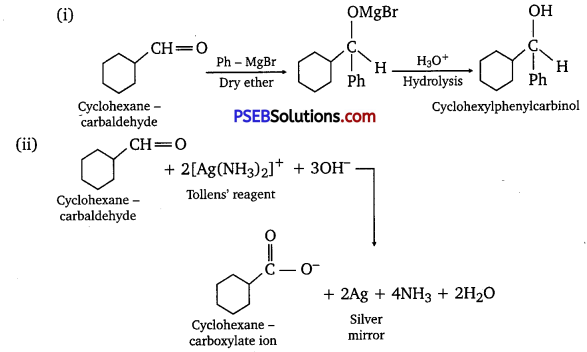
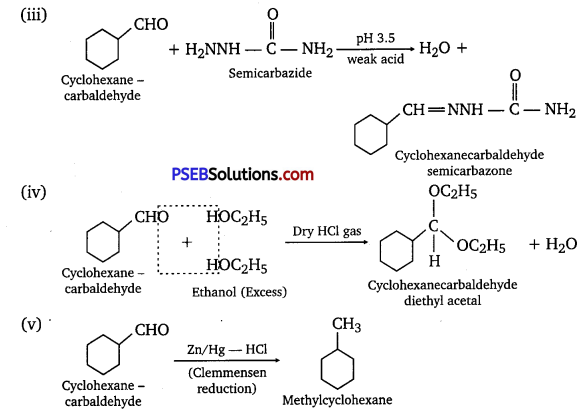
Question 7.
Which of the following compounds would undergo aldol condensation, which the Cannizzaro reaction and which neither? Write the structures of the expected products of aldol condensation and Cannizzaro reaction.
(i) Methanal
(ii) 2-Methylpentanal
(iii) Benzaldehyde
(iv) Benzophenone
(v) Cyclohexanone
(vi) 1-Phenyipropanone
(vii) Phenylacetaldehyde
(viii) Butan.1-oI
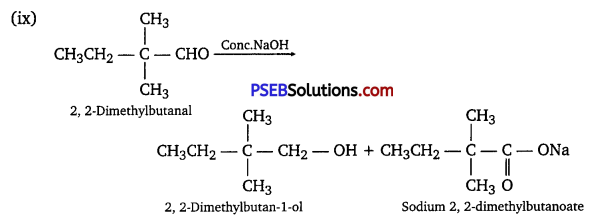
(ix) 2, 2-Dimethylbutanal
Answer:
Aldol condensation
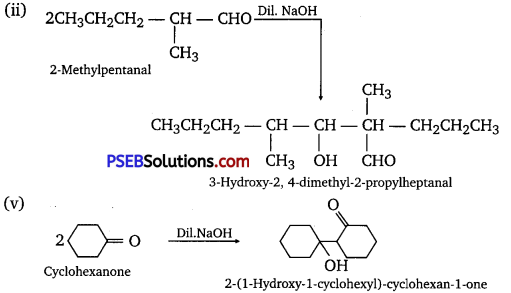
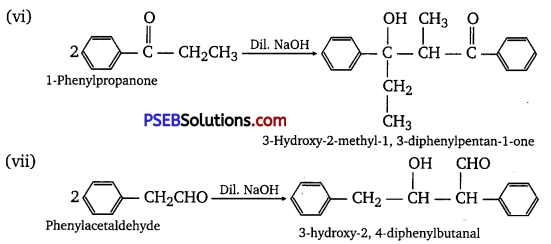
Cannizzaro reaction
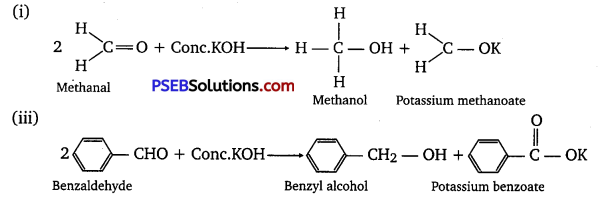
(iv) Benzophenone: It is a ketone, so it does not undergo Cannizzaro’s reaction. Without a-hydrogen, it cannot participate in aldol condensation.
(viii) Butan-1-ol: It is an alcohol. So, it cannot participate in any of the above 2 reactions.
![]()
Question 8.
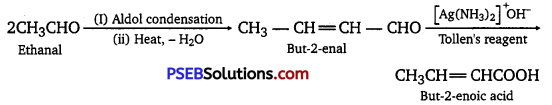
How will you convert ethanal into the following compounds?
(i) Butane-1, 3-diol
(ii) But-2-enal
(iii) But-2-enoic acid
Answer:
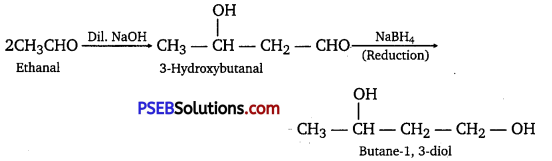
(i) On treatment with dilute alkali, ethanal produces 3-hydroxybutanal gives butane-1, 3-diol on reduction.
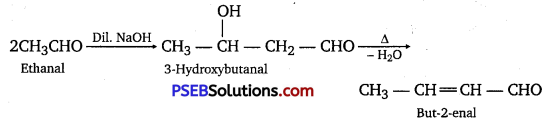
(ii) On treatment with dilute alkali, ethanal gives 3-hydroxybutanal which on heating produces but-2-enal.
(iii) When treated with Tollen’s reagent, but-2-ena produced in the above reaction produces but-2-enoic acid.
Question 9.
Write structural formulas and names of four possible aldol condensation products from propanal and butanal.
In each case, indicate which aldehyde acts as nucleophile and which as electrophile.
(i) Taking two molecules of propanal, one which acts as a nucleophile and the other as an electrophile.

(ii) Taking two molecules of butanal, one which acts as a nucleophile and the other as an electrophile.

(iii) Taking one molecule each of propanal and butanal in which propanal acts as a nucleophile and butanal acts as an electrophile.
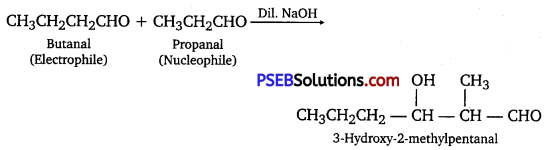
(iv) Taking one molecule each of propanal and butanal in which propanal acts as an electrophile and butanal acts as a nucleophile.

Question 10.
An organic compound with the molecular formula C9H10O forms 2, 4-DNP derivative, reduces Tollen’s reagent, and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2-benzene dicarboxylic acid. Identify the compound.
Answer:
It is given that the compound (with molecular formula C9H10O) forms 2,4-DNP derivative and reduces Tollen’s reagent. Therefore, the given compound must be an aldehyde. Again, the compound undergoes Cannizzaro reaction and on oxidation gives 1, 2-benzene dicarboxylic acid. Therefore, the -CHO group is directly attached to a benzene ring and this benzaldehyde is ortho-substituted. Hence, the compound is 2-ethyl benzaldehyde.
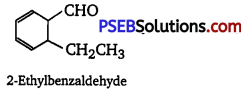
The given reactions can be explained by the following equations:
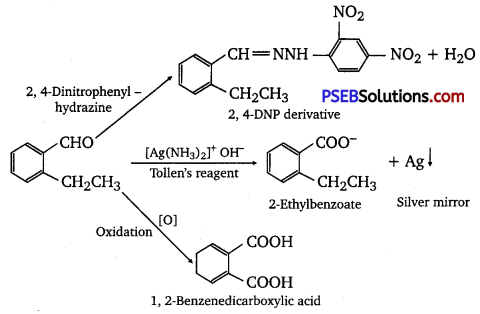
Question 11.
An organic compound (A) (molecular formula C8H16O2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid produced (B). (C) on dehydration gives but-l-ene. Write equations for the reactions involved.
Answer:
As the ester (A) with molecular formula C8H16O2 upon hydrolysis gives carboxylic acid (B) and the alcohol (C) and oxidation of (C) with chromic acid produces the acid (B), therefore, both the carboxylic acid (B) and alcohol (C) must contain the same number of carbon atoms. Now ester (A) contains eight carbon atoms, therefore, both the carboxylic acid (B) and the alcohol (C) must contain four carbon atoms each. As the alcohol (C) on dehydration gives but-l-ene, therefore, (C) must be a straight chain alcohol, i.e., butan-l-ol.
If C is butane-lol, then the acid (B) which it gives on oxidation must be butanoic acid and the ester (A) must be butyl butanoate. The relevant equations for all the reactions involved may be explained as follow :
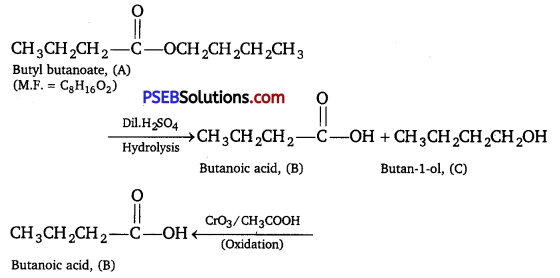
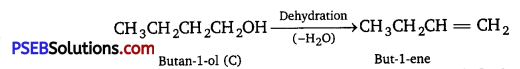
Question 12.
Arrange the following compounds in increasing order of their property as indicated:
(i) Acetaldehyde, Acetone, Di-tert-butyl ketone, Methyl tert-butyl ketone (reactivity towards HCN)
(ii) CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH,
(CH3)2CHCOOH, CH3CH2CH2COOH (acid strength)
(iii) Benzoic acid, 4-Nitrobenzoic acid, 3, 4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)
Answer:
(i) When HCN reacts with a compound, the attacking species is a nucleophile, CN–. Therefore, as the negative charge on the compound increases, its reactivity with HCN decreases. In the given compounds, the +I effect increases as shown below. It can be observed that steric hindrance also increases in the same.
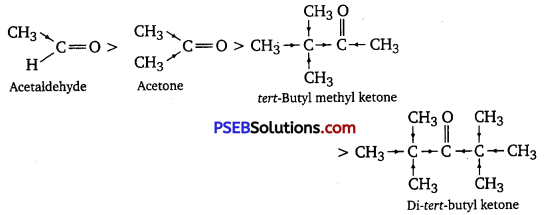
Hence, the given compounds can be arranged according to their increasing reactivities toward HCN as:
Di-tert-butyl ketone < Methyl tert-butyl ketone < Acetone < Acetaldehyde
(ii) After losing a proton, carboxylic acids gain a negative charge as shown: R – COOH →R – COO– + H+ Now, any group that will help stabilise the negative charge will increase the stability of the carboxyl ion and as a result, will increase the strength of the acid. Thus, groups having +I effect will decrease the strength of the acids, and groups having – I effect will increase the strength of the acids.
In the given compounds, -CH3 group has +I effect and Br– group has – I effect. Thus, acids containing Br– are stronger. Now, the +I effect of isopropyl group is more than that of n-propyl group. Hence, (CH3)2CHCOOH is a weaker acid than CH3CH2CH2COOH.
Also, the -I .effect grows weaker as distance increases. Hence, CH3CH(Br)CH2COOH is a weaker acid than CH3CH2CH(Br)COOH.
Hence, the strengths of the given acids increase as:
(CH3)2CHCOOH < CH3CH2CH2COOH < CH3CH(Br) CH2COOH < CH3CH2CH(Br) COOH
(iii) As we have seen in the previous case, electron-donating groups decrease the strengths of acids, while electron-withdrawing groups increase the strengths of acids. As methoxy group is an electron-donating group, 4- methoxy benzoic acid is a weaker acid than benzoic acid.
Nitro group is an electron-withdrawing group and will increase the strengths of acids. As 3,4-di nitrobenzoic acid i.ontains two nitro groups, it is a slightly stronger acid than 4-nitrobenzoic acid. Hence, the strengths of the given acids increase as:
4-Methoxybenzoic acid <Benzoic acid <4-Nitrobenzoic acid <3,4-Dinitrobenzoic acid.
![]()
Question 13.
Give simple chemical tests to distinguish between the following pairs of compounds:
(i) Propanal and Propanone
(ii) Acetophenone and Benzophenone
(iii) Phenol and Benzoic acid
(iv) Benzoic acid and Ethyl benzoate
(v) Pentan-2-one and Pentan-3-one
(vi) Benzaldehyde and Acetophenone
(vil) Ethanal and Propanal
Answer:
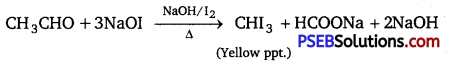
(i) Propanal and propanone:
lodoform test: This test is given by propanone and not by propanal.
Propanone on reacting with hot NaOH/I2 gives a yellow precipitate of CHI3 while propanal does not.
2NaOH + I2 → NaI + NaOI + H2O
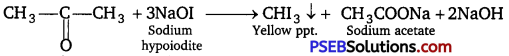
(ii) Acetophenone and benzophenone: Acetophenone responds to iodoform test, but benzophenone does not.
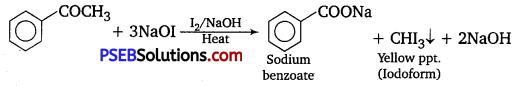
(iii) Phenol and benzoic acid: Benzoic acid reacts with NaHCO3 giving CO2 gas with effervescence, whereas phenol does not.
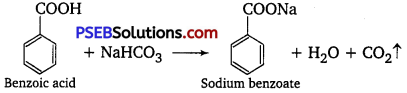
(iv) Benzoic acid and ethyl benzoate: Benzoic acid on reaction with sodium hydrogen carbonate give out CO2 gas with effervescence, while ethyl benzoate does not.
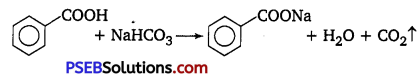
(v) Pentan-2-one and pentane-3-one: Pentan-2-one, when treated with NaOI(I2/NaOH), gives yellow precipitate of iodoform but pentane-3-one does not give this test.
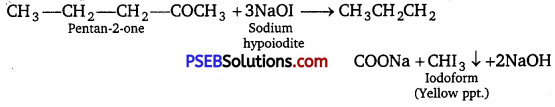
(vi) Benzaldehyde and acetophenone: Benzaldehyde “being an aldehyde gives silver mirror with Tollen’s reagent but acetophenone being a ketone does not give this test.
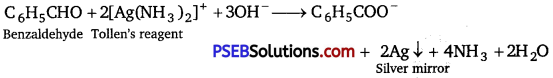
(vii) Ethanal and propanal: Ethanal responds to iodoform test, while propanal does not.
Question 14.
How will you prepare the following compounds from, benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
(i) Methyl benzoate
(ii) m -Nitrobenzoic acid
(iii) p-Nitrobenzoic acid
(iv) Phenylacetic acid
(v) p-Nitrobenz aldehyde.
Answer:
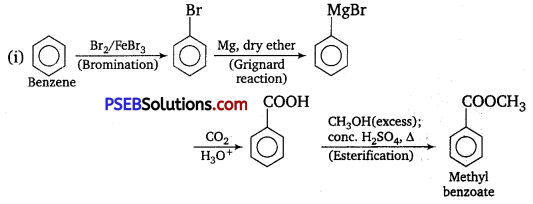
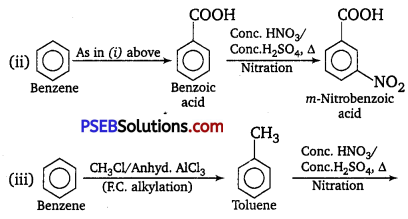
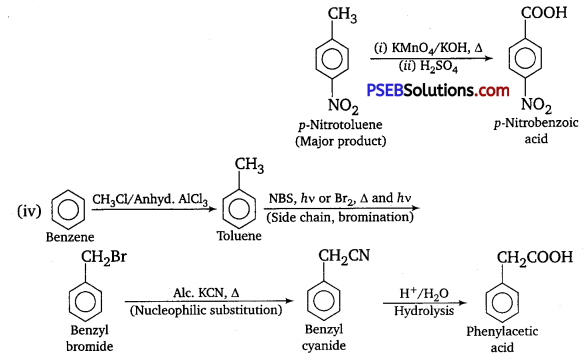
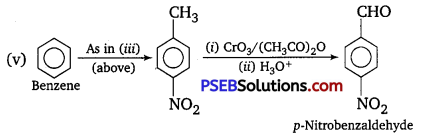
Question 15.
How will you bring about the following conversions in not more than two steps?
(i) Propanone to Propene
(ii) Benzoic acid to Benzaldehyde
(iii) Ethanol to 3-Hydroxybutanal
(iv) Benzene to m-Nitroacetophenone
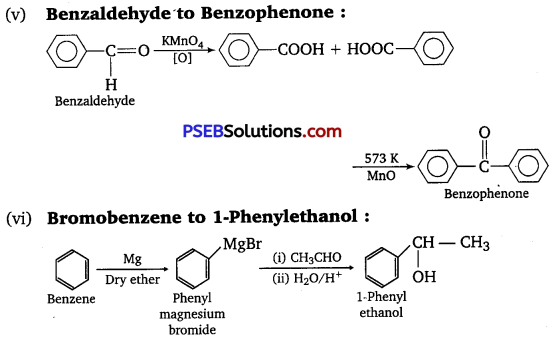
(y) Benzaldehyde to Benzophenone
(vi) Bromobenzene to 1-Phenylethanol
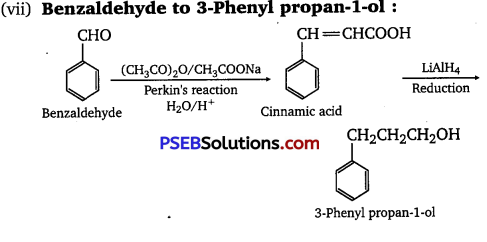
(vii) Benzaldehyde to 3-Phenylpropan-1-oI
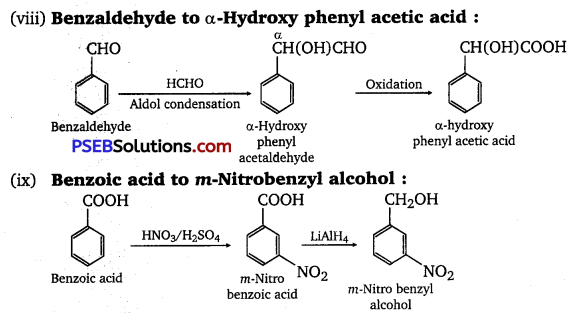
(viii) Benzaldebyde to a -Hydroxy phenylacetic acid
(ix) Benzoic acid to m-Nitro benzyl alcohol
Answer:
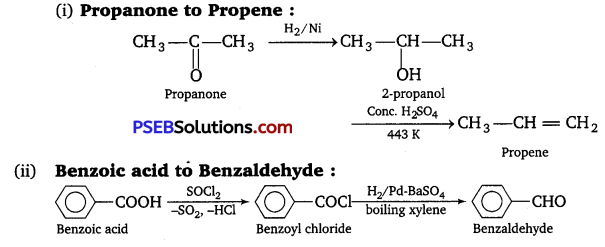
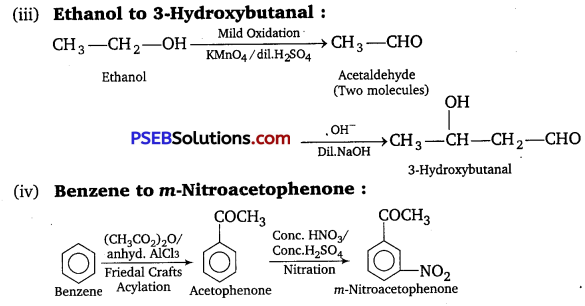
![]()
Question 16.
Describe the following:
(i) Acetylation
(ii) Cannizzaro reaction
(iii) Cross aldol condensation
(iv) Decarboxylation
Answer:
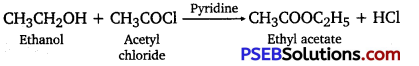
(i) Acetylation: The introduction of an acetyl functional group into an organic compound is known as acetylation. It is usually carried out in the presence of a base such as pyridine, dimethylaniline, etc. This process involves the substitution of an acetyl group for an active hydrogen atom. Acetyl chloride and acetic anhydride are commonly used as acetylating agents.
For example, acetylation of ethanol produces ethyl acetate.
(ii) Cannizzaro reaction: The self oxidation-reduction (disproportionation) reaction of aldehydes having no a-hydrogens on treatment with concentrated alkalis is known as the Cannizzaro reaction. In this reaction, two molecules of aldehydes participate where one is reduced to alcohol and the other is oxidized to carboxylic acid.
For example, when ethanol is treated with concentrated potassium hydroxide, ethanol and potassium ethanoate are produced.
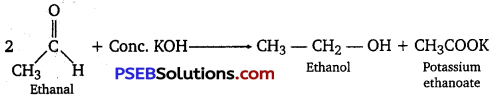
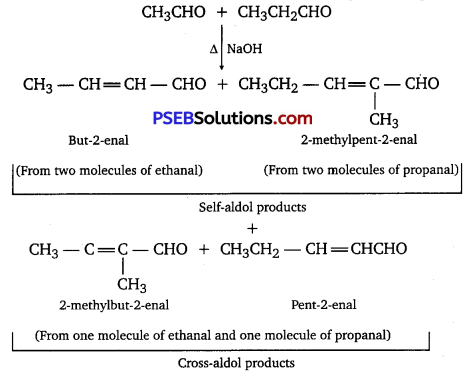
(iii) Cross-aldol condensation: When aldol condensation is carried out between two different aldehydes, or two different ketones, or an aldehyde and a ketone, then the reaction is called a cross-aldol condensation. If both the reactants contain a-hydrogens, four compounds are obtained as products. For example, ethanol and propanal react to give four products. CH3CHO + CH3CH2CHO
(iv) Decarboxylation: Decarboxylation refers to the reaction in which carboxylic acids lose carbon dioxide to form hydrocarbons when their sodium salts are heated with soda-lime (a mixture of NaOH and CaO in ratio 3:1).
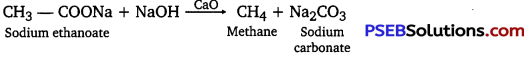
Decarboxylation also takes place when aqueous solutions of alkali metal salts of carboxylic acids are electrolyzed. This electrolytic process is known as Kolbe’s electrolysis.
Question 17.
Complete each synthesis by giving missing starting material, reagent or products
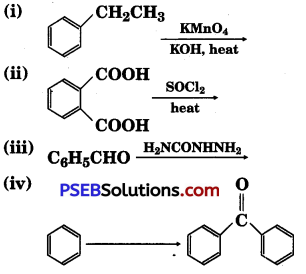
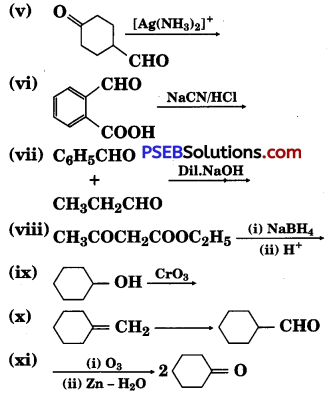
Answer:
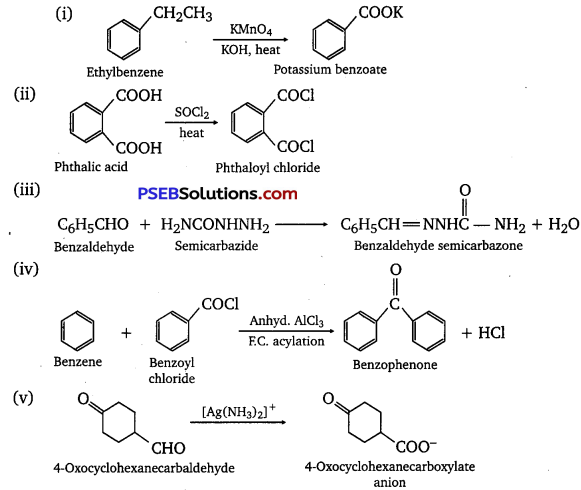
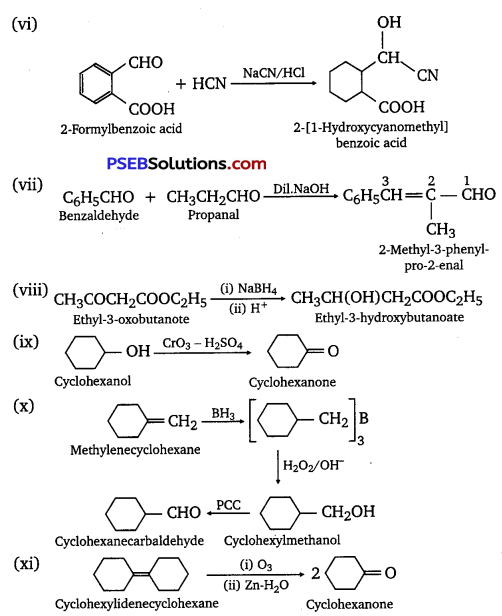
Question 18.
Give plausible explanation for each of the following:
(i) Cyclohexanone forms cyanohydrin in good yield but 2, 2, 6 trimethyl cyclohexanone does not.
(ii) There are two -NH2 groups in semicarbazide. However, only one is involved in the formation of semicarbazones.
(iii) During the preparation of esters from a carboxylic acid and an alcohol in the presence of an acid catalyst, the water or the ester should be removed as soon as it is formed.
Answer:
(i) Cyclohexanones form cyanohydrins according to the following equation :
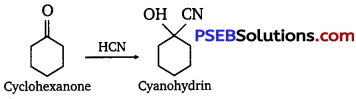
In this case, the nucleophile CN– can easily attack without any steric hindrance. However, in the case of 2, 2, 6- trimethyl cyclohexanone, methyl groups at α-positions offer steric hindrances and as a result, CN– cannot attack effectively.
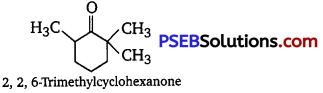
For this reason, it does not form a cyanohydrin.
(ii) Semicarbazide undergoes resonance involving only one of the two -NH2 groups, which is attached directly to the carbonyl carbon atom.
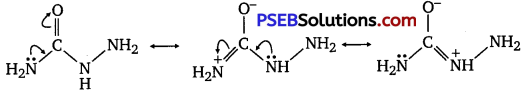
Therefore, the electron density on -NH2 group involved in the resonance also decreases. As a result, it cannot act as a nucleophile.
Since the other -NH2 group is not involved in resonance; it can act as a nucleophile and can attack carbonyl-carbon atoms of aldehydes and ketones to produce semicarbazones.
(iii) Ester along with water is formed reversibly from a carboxylic acid and an alcohol in presence of an acid.
![]()
If either water or ester is not removed as soon as it is formed, then it reacts to give back the reactants as the reaction is reversible. Therefore, to shift the equilibrium in the forward direction i.e., to produce more ester, either of the two should be removed.
![]()
Question 19.
An organic compound contain 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound Is 86. It does not reduce Tollens’ reagent but forms an additional compound with sodium hydrogen sulphite and give positive iodoform test. On vigorous oxidation, it gives ethanoic and propanoic acid. Write the possible structure of the compound.
Solution:
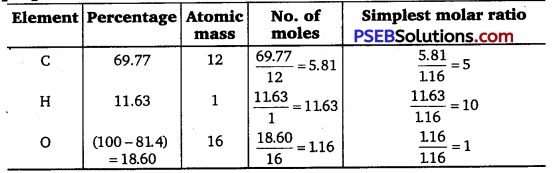
Empirical formula of the compound A = C5H10O
Molecular formula of the compound A = n (Empirical formula)
n = \(\frac{\text { Molecular mass of compound } A}{\text { Empirical formula mass of compound } A} \)
Molecular mass of compound A =86
Empirical formula mass of compound
A = 5 x 12 + 1 x 10+1 x 16
= 60+10+16 = 86
n = \(\frac{86}{86}\) = 1
Molecular formula of the compound
A = 1 (C5H10O) = C5H10O
As the compound A forms addition compound with NaHSO3 therefore it must be either an aldehyde or ketone. As it does not reduce Tollen’s reagent and give positive iodoform test therefore it must be a methyl ketone. As on oxidation, the compound A gives a mixture of ethanoic acid and propane acid, therefore compound A is
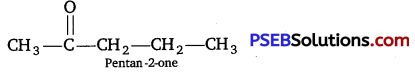
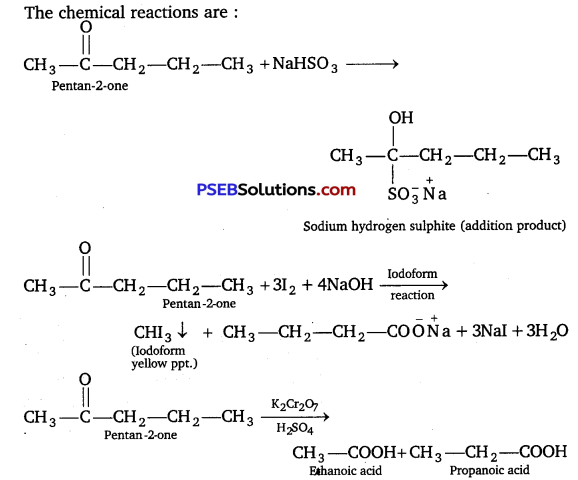
Question 20.
Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger
acid than phenol. Why?
Answer:
(i) Resonance structures of phenoxide ion are:
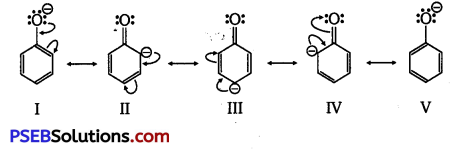
It can be observed from the resonance structures of phenoxide ion that in II, III and IV, less electronegative carbon atoms carry a negative charge. Therefore, these three structures contribute negligibly towards the resonance stability of the phenoxide ion. Hence, these structures can be eliminated. Only structures I and V carry a negative charge on the more electronegative oxygen atom.
(ii) Resonance structures of carboxylate ion are :
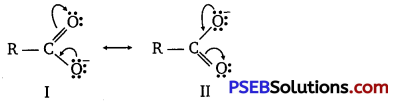
In the case of carboxylate ions, resonating structures I and II contain a charge carried by a more electronegative oxygen atom. Further, in resonating structures I and II, the negative charge is delocalized over two oxygen atoms. But in resonating structures I and V of the phenoxide ion, the negative charge is localized on the same oxygen atom. Therefore, the resonating structures of carboxylate ion contribute more towards its stability than those of phenoxide ion. As a result, carboxylate ion is more resonance-stabilized than phenoxide ion. Hence, carboxylic acid is a stronger acid than phenol.
Chemistry Guide for Class 12 PSEB Aldehydes, Ketones, and Carboxylic Acids Textbook Questions and Answers
Question 1.
Write the structures of the following compounds :
(i) α-Methoxypropionaldehyde
(ii) 3-Hydroxybutanal
(iii) 2-Hydroxycyclopentane carbaldehyde
(iv) 4-Oxopentanal
(v) Di-sec. butyl ketone
(vi) 4-Fluoroacetophenone
Answer:
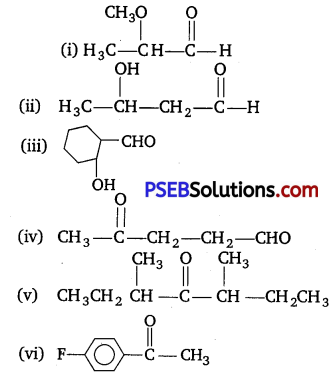
Question 2.
Write the structures of products of the following reactions;
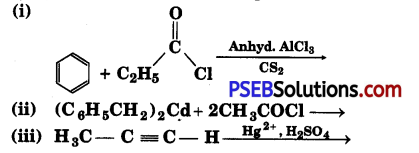
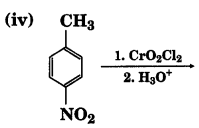
Answer:
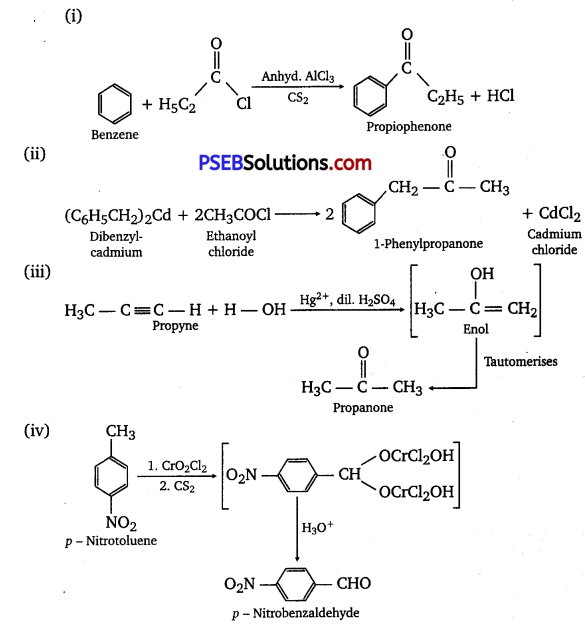
![]()
Question 3.
Arrange the following compounds in increasing order of their boiling points.
CH3CHO, CH3CH2OH, CH3OCH3, CH3CH2CH3
Answer:
Hydrocarbons are non-polar having weakest attractive forces; Ethers are polar (dipolar forces); Aldehydes have strong dipolar interactions;
Alcohols have maximum intermolecular forces due to hydrogen bonding.
CH3CH2CH3 < CH3OCH3 < CH3CHO< CH3CH2OH.
Question 4.
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
(i) Ethanal, Propanal, Propanone, Butanone.
(ii) Benzaldehyde, p-Tolualdehyde, p -Nitrobenz aldehyde, Acetophenone.
(Hint: Consider steric effect and electronic effect)
Answer:
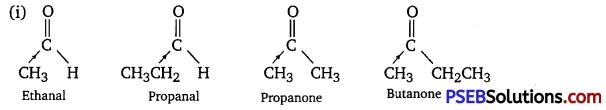
The +I effect of the alkyl group increases in the order:
Ethanal < Propanal < Propanone < Butanone
The electron density at the carbonyl carbon increases with the increase in the +I effect. As a result, the chances of attack by a nucleophile decrease. Hence, the increasing order of the reactivities of the given carbonyl compounds in nucleophilic addition reactions is:
Butanone < Propanone < Propanal < Ethanal
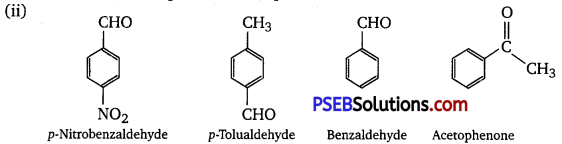
The + I effect is more in ketone than in aldehyde. Hence, acetophenone is the least reactive in nucleophilic addition reactions. Among aldehydes, the +1 effect is the highest in p-total dehyde because of the presence of the electron-donating -CH3 group and the lowest in p-nitrobenzaldehyde because of the presence of the electron-withdrawing 2 groups. Hence, the increasing order of the reactivities of the given compounds is :
Acetophenone < p – tolualdehyde <Benzaldehyde <p – Nitrobenzaldehyde
Question 5.
Predict the products of the following reactions:
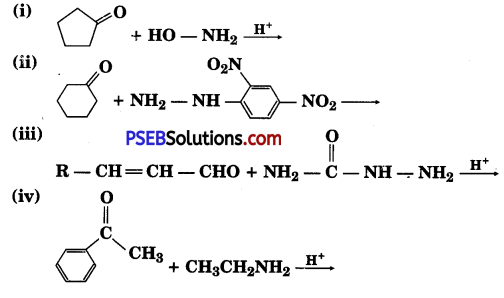
Answer:
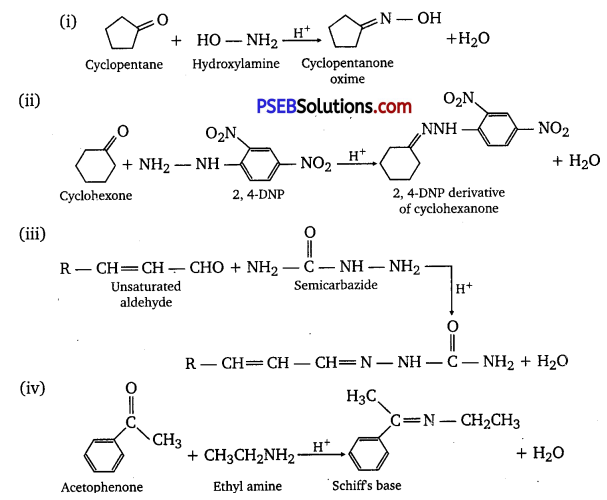
Question 6.
Give the IUPAC names of the following compounds :
(i) PhCH2CH2COOH
(ii) (CH3)2C = CHCOOH
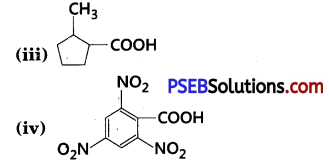
Answer:
(i) 3-Phenylpropanoic acid
(ii) 3-Methylbut-2-enoic acid
(iii) 2-Methylcyclopentanecarboxylic acid
(iv) 2,4,6-Trinitrobenzoic acid
![]()
Question 7.
Show how each of the following compounds can be converted to benzoic acid.
(i) Ethylbenzene
(ii) Acetophenone
(iii) Bromobenzene
(iv) Phenylethene (Styrene)
Answer:
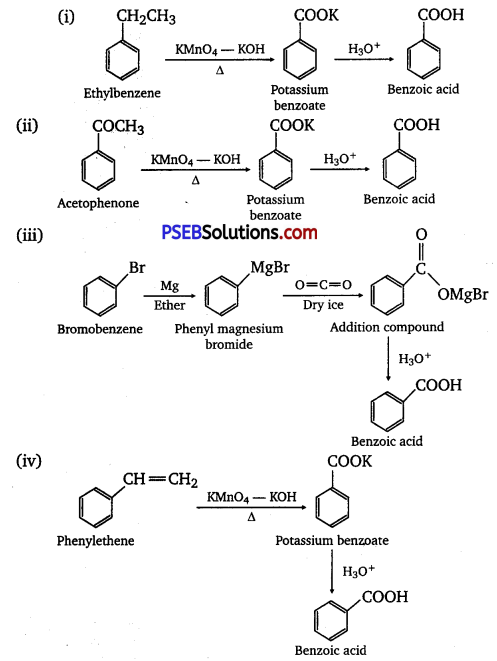
Question 8.
Which acid of each pair shown here would you expect to be stronger?
(i) CH3CO2H or CH2FCO2H
(ii) CH2FCO2H or CH2CICO2H
(iii) CH2FCH2CH2CO2H or CH3CHFCH2CO2H
![]()
Answer:

The +I effect of the -CH3 group increases the electron density on the O-H bond. Therefore, the release of protons becomes difficult. On the other hand, the – I effect of F decreases the electron density on the O-H bond. Therefore, protons can be released easily. Hence, CH2FCO2H is a stronger acid than CH3CO2H.

F has a stronger – I effect than Cl. Therefore, CH2FCO2H can release protons more easily than CH2ClCO2H.
Hence, CH2FCO2H is a stronger acid than CH2ClCO2H.
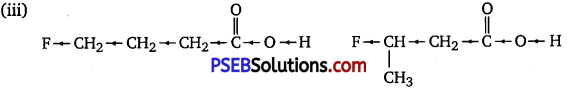
The inductive effect decreases with an increase in distance. Hence, the +I effect of F in CH3CHFCH2CO2H is more than it is in CH2FCH2CH2CO2H.
Hence, CH3CHFCH2CO2H is a stronger acid than CH2FCH2CH2CO2H.

Due to the – I effect of F, it is easier to release proton in the case of compound (A). However, in the case of compound (B), release of proton is difficult due to the +1 effect of -CH3 group. Hence, (A) is a stronger acid than (B).
