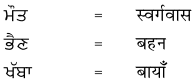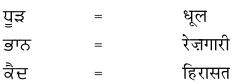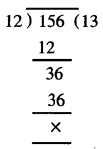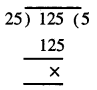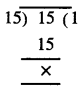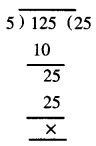Punjab State Board PSEB 7th Class Hindi Book Solutions Chapter 15 धर्मशाला Textbook Exercise Questions and Answers.
PSEB Solutions for Class 7 Hindi Chapter 15 धर्मशाला (2nd Language)
Hindi Guide for Class 8 PSEB धर्मशाला Textbook Questions and Answers
धर्मशाला अभ्यास
1. नीचे गुरुमुखी और देवनागरी लिपि में दिये गये शब्दों को पढ़ें और हिन्दी शब्दों को लिखने का अभ्यास करें :


उत्तर :
विद्यार्थी अपने अध्यापक/अध्यापिका की सहायता से स्वयं करें।

2. नीचे एक ही अर्थ के लिए पंजाबी और हिंदी भाषा में शब्द दिये गये हैं। इन्हें ध्यान से पढ़ें और हिंदी शब्दों को लिखें :


उत्तर :
विद्यार्थी अपने अध्यापक/अध्यापिका की सहायता से स्वयं करें।
3. इन प्रश्नों के उत्तर एक या दो वाक्यों में लिखें :
(क) धर्मशाला किस पर्वतमाला की गोद में बसा है ?
उत्तर :
धर्मशाला धौलाधार पर्वतमाला की गोद में बसा है।
(ख) समुद्रतल से धर्मशाला की ऊँचाई कितनी है?
उत्तर :
समुद्र तल से धर्मशाला की ऊँचाई 4500 फुट है।
(ग) मक्लोडगंज में घूमते हुए लेखिका को ल्हासा की याद क्यों आ गयी?
उत्तर :
मक्लोडगंज में घूमते हुए लेखिका को ल्हासा की याद इसलिए आ गई क्योंकि वहाँ सभी दुकानें तिब्बतियों की थीं। अधिकतर दुकानें ऊनी वस्त्रों, स्वैटरों, सजावटी वस्तुओं तथा हस्तकला की थीं जैसी उसने कभी ल्हासा में देखी थीं।।

(घ) मक्लोडगंज की मुख्य विशेषता क्या है ?
उत्तर :
मक्लोडगंज की मुख्य विशेषता मन्दिर में स्थापित महात्मा बुद्ध की विशाल प्रतिमा तथा बुद्ध पोथियों का संग्रहण है। तिब्बती शासक दलाई लामा का वह अस्थाई मुख्यालय है।
(ङ) भागसू नाग क्यों प्रसिद्ध है?
उत्तर :
भागसू नाग पत्थरों पर उकेरी गई नाग की मूर्तियों के कारण प्रसिद्ध है।
(च) वार मैमोरियल का भारतीय इतिहास में क्या महत्त्व है?
उत्तर :
वार मैमोरियल का हिंदी अर्थ युद्ध स्मारक है। इसका निर्माण उन वीर सैनिकों की याद में किया गया है जिन्होंने देश की रक्षा के लिए अपने प्राण न्योछावर कर दिए। यह स्मारक कर्त्तव्य – पथ पर अपने जीवन का बलिदान करने वाले जवानों को नमन करता है।
(छ) चिन्मय तपोवन क्यों प्रसिद्ध है?
उत्तर :
चिन्मय तपोवन हनुमान जी की नौ मीटर ऊँची मूर्ति, राम मंदिर तथा अपने संस्थापक चिन्मयानन्द के कारण प्रसिद्ध है।
(ज) अंदरेटा में लेखिका को किन कलाकारों के चित्रों ने आकर्षित किया?
उत्तर :
अंदरेटा में लेखिका को श्रेष्ठ चित्रकार सोभा सिंह के चित्रों ने अपनी ओर आकर्षित किया।
(झ) पालमपुर के किन-किन वीर सैनिकों ने कारगिल युद्ध के दौरान अपनी शहादत दी?
उत्तर :
कारगिल युद्ध के दौरान जिन जवानों की शहादत से विजय प्राप्त हुई उनमें कैप्टन विक्रम बतरा, मेजर सुधीर वालिया तथा कैप्टन सौरभ कालिया पालमपुर के थे।

4. इन प्रश्नों के उत्तर चार या पाँच वाक्यों में लिखें :
(क) मक्लोडगंज का ऐतिहासिक महत्त्व क्या है?
उत्तर :
मक्लोडगंज तिब्बती शासक दलाई लामा का अस्थाई मुख्यालय है। सन् 1959 ई० में जब चीन ने तिब्बत देश पर आक्रमण द्वारा कब्जा कर लिया था तब दलाई लामा सहित 85,000 निर्वासित बौद्ध तिब्बतियों को भारत ने धर्मशाला में मकलोडगंज में शरण दी थी। यहाँ मंदिर में महात्मा बुद्ध की विशाल प्रतिमा तथा बुद्ध पोथियाँ संग्रहित हैं। यह तिब्बत की राजधानी ल्हासा के समान देखने में लगता है।।
(ख) पर्वतीय स्थल हमें क्यों आकर्षित करते हैं?
उत्तर :
पर्वतीय स्थल प्राकृतिक सौंदर्य के दर्शनों के लिए जाने जाते हैं। यही इनके आकर्षण का केन्द्र भी है। पर्वतीय स्थलों पर पर्वत श्रृंखलाओं को देखना अपने आप में एक अद्भुत दृश्य है। यहाँ झील अपने अद्भुत सौन्दर्य के कारण पर्यटकों के लिए आकर्षण का केन्द्र है। इसका जल शीतल तथा पीने योग्य है। यहाँ फलों तथा चाय के बागानों की सुगंध वातावरण को मोहक बना देती है। यहाँ झील में भ्रमण करने के लिए नौकाएँ उपलब्ध रहती हैं। पर्यटकों के रहने के लिए उचित व्यवस्थाएँ भी हैं। अपने इन्हीं प्राकृतिक दृश्यों से परिपूर्ण सौंदर्य के कारण पर्वतीय स्थल हमें अपनी ओर खींचते हैं।
5. इन शब्दों का वाक्यों में प्रयोग करें :
- पर्वतीय
- हस्तकला
- अनगिनत
- निर्वासित
- शहादत
उत्तर :
- पर्वतीय – पर्वतीय स्थल पर्यटकों को अपनी ओर खींचते हैं।
- हस्तकला – हिमाचल के बाजारों में अधिकतर दुकानें हस्तकला से जुड़ी वस्तुओं की हैं।
- अनगिनत – अनगिनत प्राणियों की देखभाल परमात्मा ही तो करते हैं।
- निर्वासित – निर्वासित बौद्ध तिब्बतियों को भारत ने शरण दी थी।
- शहादत – हमें अपने वीर सैनिकों की शहादत को कभी नहीं भूलना चाहिए।

6. इन शब्दों को जोड़कर नया शब्द लिखें :
- सूर्य + उदय
- सूर्य + अस्त
- मुख्य + आलय
- स्वर्ण + अक्षर
- हिम + आच्छादित
उत्तर :
- सूर्य + उदय = सूर्योदय
- सूर्य + अस्त = सूर्यास्त
- मुख्य + आलय = मुख्यालय
- स्वर्ण + अक्षर = स्वर्णाक्षर
- हिम + आच्छादित = हिमाच्छादित
7. इन शब्दों और शब्दांशों को मिलाकर नया शब्द लिखें :
- पर्वत + ईय
- निर् + वास + इत
- प्र + शासन + इक
- प्रकृति + इक
- भारत + ईय
- अंक + इत
उत्तर :
- पर्वत + ईय = पर्वतीय
- निर् + वास + इत = निर्वासित
- प्र + शासन + इक = प्रशासनिक
- प्रकृति + इक = प्राकृतिक
- भारत + ईय = भारतीय
- अंक + इत = अंकित

प्रयोगात्मक व्याकरण
- सामिषा अपने माता-पिता के साथ धर्मशाला गयी।
- डल झील के चारों ओर देखो देवदार के पेड़ हैं।
- घरों के सामने बाँस के अनगिनत वृक्ष हैं।
- हम बागानों, मकानों और बंगलों के बीच से गुजरती सड़क से न्यूगल कैफेटेरिया पहुँचे।
- कैफेटेरिया के पीछे न्यूगल खड्ड है।
- हम खराब सड़क के कारण ट्रैकिंग स्थल त्रिपुंड नहीं जा सके।
- मेरे सामने प्रकृति के अद्भुत दृश्य थे।
उपर्युक्त वाक्यों में के साथ, के चारों ओर, के सामने, के बीच, के पीछे, के कारण तथा सामने शब्द संज्ञा तथा सर्वनाम शब्दों के साथ आकर उनका संबंध वाक्य के दूसरे शब्दों के साथ बता रहे हैं। अतः ये संबंध बोधक है।
अतएव जो शब्द संज्ञा या सर्वनाम शब्दों के साथ जुड़कर उनका संबंध वाक्य के दूसरे शब्दों से बताते हैं, उन्हें संबंध बोधक कहते हैं।
यदि इन संबंध बोधक अविकारी शब्दों को वाक्य में से निकाल दिया जाये तो वाक्य का अर्थ ही नहीं रहता।
अन्य संबंध बोधक शब्द : पहले, बाद, आगे, पीछे, बाहर, भीतर, ऊपर, नीचे, पास, अनुसार, तरह, समान, बिना, कारण, तक, भर, संग, साथ, के मारे, बगैर, रहित, सिवाय आदि।
संबंध बोधक का प्रयोग दो प्रकार से होता है :
- विभक्तियों के साथ
- विभक्तियों के बिना
1. विभक्तियों के साथ संबंध बोधक शब्द प्रमुख रूप से निम्नलिखित तरह से प्रयुक्त होते हैं:
- हमने चिन्मय तपोवन की ओर प्रस्थान किया।
- वीर सैनिकों ने देश की रक्षा के लिए प्राण न्योछावर कर दिये।
- पालम की घाटी सुन्दरी की तरह प्रतीत होती है।

2. विभक्तियों के बिना संबंध बोधक शब्द इस प्रकार प्रयुक्त होते हैं:
- मैं जीवन भर इस यात्रा को याद रखूगी
- ज्ञान बिना जीवन बेकार है।
- मुझे कई दिनों तक घर की याद नहीं आयी।
- सड़क पर काली सफेद लकीर लगायी गयी है।
धर्मशाला Summary in Hindi
धर्मशाला पाठ का सार
‘धर्मशाला’ पाठ की लेखिका डॉ० मीनाक्षी वर्मा हैं। लेखिका इस पाठ के माध्यम से यह बताना चाहती है कि यात्राओं से मनोरंजन, ज्ञानवर्धन एवं अज्ञात स्थलों की जानकारी के साथ – साथ भाषा और संस्कृति का आदान – प्रदान भी होता है। इस लेख में भारत के हिमाचल राज्य के शहर धर्मशाला की यात्रा का वर्णन किया गया है।

सामिषा अपने माता – पिता और भाई साराँश के साथ धर्मशाला पर्वतीय स्थान पर घूमने आई हुई है। यहाँ वे हिमाचल पर्यटन के होटल ‘धौलाधार’ में ठहरे हुए हैं। धौलाधार सदैव बर्फ से ढकी रहती है। वह पर्वतमाला की गोद में बसी है। सामिषा को डायरी लिखने का शौक है। वह प्रतिदिन जहाँ भी जाती है, रात को सोने से पहले अपनी डायरी में लिख देती 27 दिसम्बर, सन् 2011 सामिषा अपनी डायरी में लिखती है कि वे लोग सुबह होते ही अपनी कार में बैठ कर धर्मशाला घूमने निकल पड़े। धर्मशाला समुद्र तल से 4500 फुट की ऊँचाई पर था। धौलाधार पर्वत श्रेणी की तलहटी में बसा खूबसूरत शहर था।
धर्मशाला दो हिस्सों में बँटा हुआ था। लोअर धर्मशाला और अपर धर्मशाला। लोअर धर्मशाला में प्रशासनिक इकाइयाँ हैं तो अपर धर्मशाला में सैनिक छावनी, सेंट जोन चर्च तथा मक्लोडगंज में तिब्बती कालोनियाँ हैं। सामिषा और उसका परिवार सबसे पहले धर्मशाला से 10 कि०मी० दूर मक्लोडगंज घूमने गए। मार्ग में उसे प्रकृति के सुंदर – सुंदर दृश्य दिखाई दिए। वह हरी भरी घाटियों को बिना पलक झपकाए निहार रही थी।
ठीक दस बजे सामिषा परिवार सहित मक्लोडगंज पहुँच गई। यहाँ उनके सामने सुंदर पर्वत मालाएँ थीं। बाज़ार में घूमते हुए उन्होंने देखा कि यहाँ सभी दुकानें तिब्बतियों की हैं। हस्तकला तथा ऊनी वस्त्रों की दुकानों पर भरमार थी। सामिषा को घूमते हुए ऐसा लग रहा था मानो वे ल्हासा में घूम रही हो।
सामिषा ने यहाँ बौद्ध मंदिरों के दर्शन किए जिन पर बुद्ध की विशाल प्रतिमाएँ तथा बुद्ध पोथियाँ संग्रहित थी। मक्लोडगंज को तिब्बती शासक दलाई लामा का अस्थाई मुख्यालय माना जाता है। सन् 1959 ई० में चीन ने जब तिब्बत देश पर आक्रमण कर उसे अपने कब्जे में कर लिया था तब दलाईलामा सहित 85000 निर्वासित बौद्ध तिब्बतियों को भारत ने धर्मशाला में मक्लोडगंज में शरण दी थी।

इसके बाद सामिषा ने यहाँ से 15 कि०मी० दूर भागसू में स्थित शिव मंदिर तथा जल प्रपात देखे तथा इसके बाद वे सीधे पवित्र डल झील गए जो एक पिकनिक स्थल भी है।
28 दिसम्बर, सन् 2011 सामिषा और उसका परिवार सुबह सवेरे तैयार होकर युद्ध स्मारक देखने चल पड़े। इसका निर्माण युद्धों में देश की रक्षा के लिए अपने प्राणों की आहुति देने वाले शहीद जवानों की याद में किया गया था। शहीद हुए सैनिकों के नाम काले संगमरमर की तीन पट्टिकाओं पर लिखे हुए थे।
ये नाम आने वाली पीढ़ी के लिए मार्गदर्शन का काम करने वाला था कि कर्त्तव्य पथ पर अपने जीवन का बलिदान करने से हमें पीछे नहीं हटना चाहिए। इसके कुणाल पत्थरी देखने गए तथा तत्पश्चात् यहाँ से दस कि०मी० दूर चिन्मय तपोवन की ओर चल पड़े। इस तपोवन की स्थापना चिन्मयानंद द्वारा की गई थी।
29 दिसम्बर, सन् 2011 सुबह सवेरे सामिषा और उसके परिवार ने धर्मशाला से 17 कि०मी० दूर त्रिपुंड जाने का मन बनाया लेकिन अधिक बर्फबारी के कारण वे वहाँ न जा सके, तब वे घूमने पालमपुर चले गए। पालमपुर काँगड़ा की मुख्य घाटी है। यहाँ घरों के सामने अनगिनत झुरमुट दिखाई देते हैं। यहां चाय के बागानों तथा बंगलों के बीच गुजरने वाली सड़क से होते हुए सामिषा और उसका परिवार न्यूगल कैफेटेरिया पहुंचे।
यहाँ न्यूगल नामक बहुत गहरी खड्ड है जिसमें पहाड़ से टूटी चट्टानों के टुकड़े हैं। खड्ड में बहने वाला साफ़ सुथरा पानी धौलाधार की बर्फ से पिघल कर आता है। इसके बाद वे सभी पालमपुर से 13 कि०मी० दूर अंदरेटा गाँव की ओर चल पड़े। अंदरेटा कई कलाकारों का निवास स्थान रहा है। जिनमें चित्रकार सोभा सिंह तथा नाटककार नोरा रिचर्डस का नाम प्रमुख है।
श्री गुरु नानक देव जी का चित्र जो सोभा सिंह द्वारा तैयार किया गया था प्रत्येक सिक्ख परिवार में है। पालमपुर का नाम कला प्रेमियों के साथ – साथ वीर सैनिकों से भी जुड़ा है। कारगिल युद्ध के दौरान शहीद हुए कैप्टन विक्रम बतरा, मेजर सुधीर वालिया तथा कैप्टन सौरभ कालिया का सम्बन्ध पालमपुर से ही है।

मेजर सुधीर वालिया को अशोक चक्र से तथा कैप्टन विक्रम बतरा को बहादुरी के लिए परमवीर चक्र से सम्मानित किया गया। अपनी यात्रा के अंत में सामिषा परिवार सहित बैजनाथ में भगवान शिव के दर्शन कर अपनी यात्रा की सुखद यादें और अनुपम प्राकृतिक सौन्दर्य का आनन्द लेते घर वापिस आ गए।
धर्मशाला कठिन शब्दों के अर्थ
- देवभूमि = देवताओं की भूमि।
- पर्वत की तलहटी = पहाड़ का धरातल।
- पर्वतीय स्थल = पहाड़ी क्षेत्र।
- खूबसूरत = सुंदर।
- लोअर = नीचे का।
- अपर = ऊपर का।
- प्रशासनिक = सरकारी दफ्तर जो प्रशासन से सम्बंधित हों।
- भ्रमण = घूमना।
- झुरमुट = समूह।
- सीढ़ीनुमा = सीढ़ी के आकार का।
- वेग से = तेज़ गति से।
- अपलक = बिना पलक झपकाए।
- निहारना = देखना।
- प्रतिमा = मूर्ति।
- हिमाच्छादित = बर्फ को छेदते हुए।
- हस्तकला = हाथ की कला।
- रेस्तराँ = खाने – पीने की जगह।
- पोथियाँ = धार्मिक पुस्तकें।
- लिप्त = शामिल।
- जलाशय = तालाब।
- जलप्रपात = झरने।
- भागसू = जल।
- पाषाण = पत्थर।
- साँझ = शाम।
- वार = लड़ाई।
- पथ = रस्ता।
- स्वर्णाक्षरों = सोने के अक्षरों से।
- मनभावन = मन को भा जाने वाले।
- खड़ = खाई।
- पौ फटते ही = सूर्य की प्रथम किरण निकलते ही।
- संग्रहालय = कृतियों एवं विशेष वस्तुओं को रखने का स्थान।
- निर्मित = बनाया हुआ।
- प्राचीन = पुराना।
- शहादत = बलिदान।

धर्मशाला गद्यांशों की सप्रसंग व्याख्या
1. हम 10 बजे मक्लोडगंज पहुँच गए। सामने हिमाच्छादित पर्वतों को देखकर हमारे मन में उमंगें करवटें लेने लगीं। हमने यहाँ के बाज़ार में घूमते हुए देखा कि यहाँ सभी दुकानें तिब्बयों की हैं। अधिकतर दुकानें ऊनी वस्त्रों, स्वैटरों, सजावटी वस्तुओं तथा हस्तकला की हैं तो कुछ तिब्बती रेस्तराँ हैं। यहाँ हमें ऐसे प्रतीत हो रहा था मानों हम ल्हासा (तिब्बत की राजधानी ) में भ्रमण कर रहे हों। हमने यहाँ तिब्बतियों द्वारा स्थापित बौद्ध मन्दिर के भी दर्शन किए। मन्दिर में महात्मा बुद्ध की विशाल प्रतिमा तथा बुद्ध पोथियाँ संग्रहित हैं।
प्रसंग – प्रस्तुत गद्यांश हमारी हिंदी की पाठ्य पुस्तक में संकलित ‘धर्मशाला’ नामक शीर्षक से उदधुत है जिसकी लेखिका डॉ० मीनाक्षी वर्मा हैं। लेखिका ने यहाँ धर्मशाला के प्राकृतिक सौंदर्य के साथ – साथ वहाँ के बाजारों तथा वहाँ के निवासियों की धार्मिक आस्थाओं का सुंदर वर्णन किया है।
व्याख्या – लेखिका मक्लोडगंज की सुंदरता का वर्णन करते हुए कहती है कि वे अगले दिन दस बजे मक्लोडगंज पहुँच गए। सामने उसने चित्र देखा उसे वह शब्दों के माध्यम से नहीं बता सकती। बर्फ के बीच से पर्वतों को देखते हुए उनके मन में तरह – तरह की उमंगें करवट लेने लगीं। लेखिका ने वहाँ के बाज़ाराों में घूमते हुए देखा कि यहाँ अधिकतर दुकानें तिब्बतियों की हैं।
इन दुकानों में ऊनी वस्त्र, सजावटी वस्तुएँ तथा हस्तकला की वस्तुओं की भरमार थी। वहाँ एक रेस्तरां भी था जो तिब्बती था। सारे क्षेत्र को देखकर लेखिका को लग रहा था कि जैसे वह तिब्बत की राजधानी लहासा में घूम रही हो। उन्होंने मक्लोडगंज में तिब्बतियों द्वारा स्थापित किए बौद्ध मन्दिर में जाकर दर्शन किए। मन्दिर में भगवान महात्मा बुद्ध एक बहुत बड़ी मूर्ति थी। वहाँ भगवान बुद्ध से सम्बंधित अनेक पोथियाँ भी संग्रहित कर रखी थी।
विशेष –
- लेखिका ने मक्लोडगंज के बाजारों की सुंदरता तथा वहाँ स्थापित बौद्ध मन्दिर का सुंदर अंकन किया है।
- भाषा – शैली सरल तथा सहज है।
2. आज सुबह हम लोग तैयार होकर वार मैमोरियल (युद्ध स्मारक) देखने चल पड़े। इसका निर्माण उन वीर सैनिकों की याद में किया गया है जिन्होंने 1947 1948, 1962, 1965, 1971 के युद्धों में देश की रक्षा के लिए अपने प्राण न्योछावर कर दिए। काले संगमरमर की तीन पट्टिकाओं पर शहीद हुए सैनिकों के नाम अंकित हैं। यह स्मारक कर्तव्य पथ पर अपने जीवन का बलिदान करने वाले जवानों को नमन करता है तथा आने वाली पीढ़ी कोभी बताता है कि ये वे नाम हैं। जिन्हें भारतीय इतिहास में स्वर्णाक्षरों में लिखा गया है, कभी भुलाया नहीं जा सकता।
प्रसंग – प्रस्तुत पंक्तियाँ हमारी हिन्दी की पाठ्य पुस्तक के ‘धर्मशाला’ नामक यात्रा वृत्तांत से ली गई हैं। इसकी लेखिका डॉ मीनाक्षी वर्मा हैं। इस पाठ में उन्होंने धर्मशाला के प्राकृतिक सौंदर्य के साथ – साथ वहाँ के निवासियों की धार्मिक आस्थाओं तथा वहाँ के युद्ध स्मारक का सजीव चित्रण किया है।

व्याख्या – लेखिका युद्ध स्मारक का वर्णन करते हुए कहती है कि आज सुबह – सुबह वे लोग तैयार होकर युद्ध स्मारक देखने चल पड़े। इस युद्ध स्मारक का निर्माण सन् 1947, 1948, 1962, 1965 तथा 1971 के युद्धों में देश की रक्षा के लिए शहीद हुए उन वीर जवानों की याद में किया गया था जिन्होंने देश के लिए अपने प्राणों को न्योछावर कर दिया था। यहाँ शहीद हुए सैनिकों का नाम काले संगमरमर की तीन पटिकाओं पर लिखा हुआ है।
यह स्मारक हमें उन जवानों को नमन करने की याद दिलाता है जिन्होंने अपने कर्तव्य को निभाने के लिए अपने प्राणों को देश के लिए बलिदान कर दिया था। ये संगमरमर की पटिकाओं पर लिखे नाम हमें तथा आने वाली पीढ़ी को बताते हैं कि इन वीरों का नाम स्वर्णाक्षरों में लिखा है न तो इन वीरों को भुलाया जा सकता है न ही इनकी वीरता को कभी भी भुलाया जा सकेगा।
विशेष –
- लेखिका ने युद्ध स्मारक तथा देश की रक्षा हेतु अपना बलिदान देने वाले शहीदों को नमन करने की बात कही।
- भाषा शैली सरस और सुबोध है।
3. इसके उपरान्त हम पालमपुर से 13 कि०मी० दूर अंदरेटा गाँव की ओर रवाना हुए। अंदरेटा कई एकान्तप्रिय कलाकारों का निवास स्थान रहा है जिनमें चित्रकार सोभा सिंह तथा नाटककार नोरा रिचर्डस का नाम प्रमुख है। हमने पंजाब के श्रेष्ठ चित्रकार सोभा सिंह की कलाकृतियों का संग्रहालय देखा। सोभा सिंह का श्री गुरु नानक का चित्र हर सिक्ख परिवार में तथा सोहनी महीवाल का प्रसिद्ध चित्र उत्तरी भारत के हर कला प्रेमी के पास है। सोभा सिंह द्वारा निर्मित सुन्दरियों के चित्र, भारतीय चित्रकला में विशेष महत्त्व रखते हैं।
प्रसंग – प्रस्तुत गद्यांश हमारी हिन्दी की पाठ्य पुस्तक में वर्णित यात्रा वृत्तांत ‘धर्मशाला’ नामक शीर्षक से लिया गया है। इसकी लेखिका डॉ० मीनाक्षी वर्मा हैं। इस पाठ में लेखिका ने धर्मशाला के प्राकृतिक सौन्दर्य के साथ – साथ वहाँ के प्रसिद्ध व्यक्तियों एवं कलाओं का वर्णन किया है।
व्याख्या – लेखिका कहती है कि पालमपुर में घूमने के बाद वह सपरिवार पालमपुर से तेरह कि०मी० दूर अंदरेटा गाँव गए। अंदरेटा कला की दृष्टि से काफ़ी प्रसिद्ध स्थान है। यह कई एकांत चाहने वाले कलाकारों का घर रहा है। जिनमें मुख्य रूप से चित्रकार सोभा सिंह तथा प्रसिद्ध नाटककार नोरा रिचर्डस का नाम आता है।
यहाँ घूमते हुए लेखिका ने पंजाब के सुप्रसिद्ध श्रेष्ठ चित्रकार सोभा सिंह की कलाकृतियों का एक संग्रहालय देखा जिसमें उन्होंने अपनी बहुत – सी सुंदर चित्रकलाकृतियाँ रखी हुई थीं। सोभा सिंह के द्वारा बनाया गया गुरु नानक देव जी का चित्र आज सभी सिक्ख परिवारों में देखने को मिलता है।

इसके साथ – साथ सोहनी महीवाल का चित्र उत्तरी भारत के प्रत्येक कला – प्रेमी के पास मिलता है। भारतीय चित्रकला में सोभा सिंह द्वारा बनाए गए सुंदर स्त्रियों के चित्र अपना एक विशेष स्थान एवं महत्त्व रखते हैं।
विशेष –
- लेखिका ने श्रेष्ठ चित्रकार सोभा सिंह की सुंदर चित्रकला की विशेषताओं को प्रकट किया है।
- भाषा प्रवाहमयी है।
4. पालमपुर का नाम केवल कला प्रेमियों से ही नहीं जुड़ा बल्कि देश के वीर सैनिकों से भी जुड़ा है। कारगिल युद्ध के दौरान जिन जवानों की शहादत से विजय प्राप्त हुई उनमें कैप्टन विक्रम बतरा, मेजर सुधीर वालिया तथा कैप्टन सौरभ कालिया का सम्बन्ध पालमपुर से ही है। इनमें कैप्टन विक्रम बतरा की बहादुरी के लिए सर्वश्रेष्ठ पुरस्कार परमवीर चक्र तथा मेजर सुधीर वालिया को अशोक चक्र से सम्मानित किया गया है।
प्रसंग – प्रस्तुत गद्यांश हमारी हिन्दी की पाठ्य पुस्तक में वर्णित यात्रा वृतांत ‘धर्मशाला’ नामक पाठ से लिया गया है, जिसकी लेखिका डॉ० मीनाक्षी वर्मा हैं। लेखिका ने इस पाठ में प्राकृतिक सौन्दर्य के साथ – साथ वहाँ के वीर जवानों की वीरता का सुंदर चित्रण किया है।
व्याख्या – लेखिका कहती है कि पालमपुर पर्वतीय क्षेत्र का नाम मात्र कला प्रेमियों से ही नहीं जुड़ा है अपितु इसका नाम भारत देश के साहसी सैनिकों से भी जुड़ा है। कारगिल युद्ध में भारत को जिन वीर जवानों की शहादत से भारत को विजय प्राप्त हुई उनमें कैप्टन विक्रम बतरा, मेजर सुधीर वालिया तथा कैप्टन सौरभ कालिया पालमपुर के ही रहने वाले थे।
इन वीर जवानों में से कैप्टन विक्रम बतरा को वीरता के लिए भारतीय सेना का सबसे बड़ा पदक परमवीर चक्र मिला तथा मेजर सुधीर वालिया को अशोक चक्र से सम्मानित किया गया। भाव यह है कि पालमपुर कलाप्रेमियों के साथ – साथ वीर बाँकुरों का भी स्थान है।

विशेष –
- लेखिका ने पालमपुर को कला तथा वीरता का अनूठा सामंजस्य स्थापित करने वाला क्षेत्र बताया है।
- भाषा शैली अत्यंत सरल और सुबोध है।
![]()
![]()
![]()
![]()
![]()
![]()
![]()