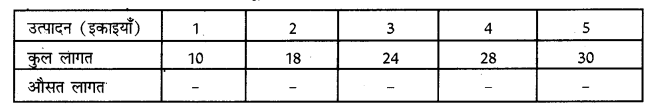
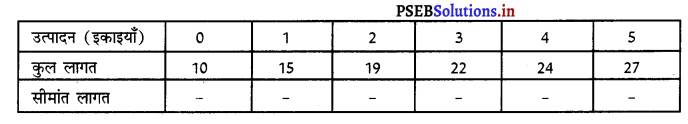
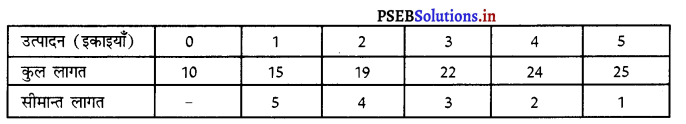
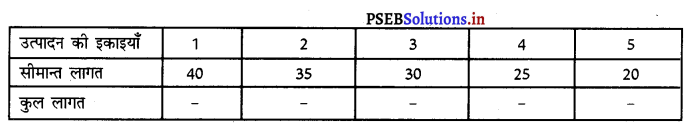
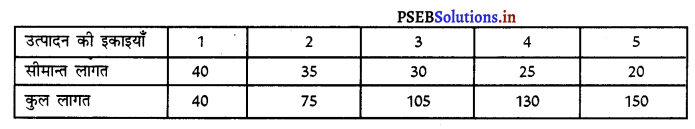
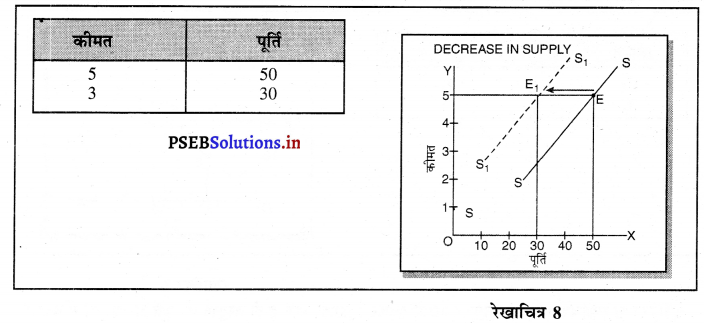
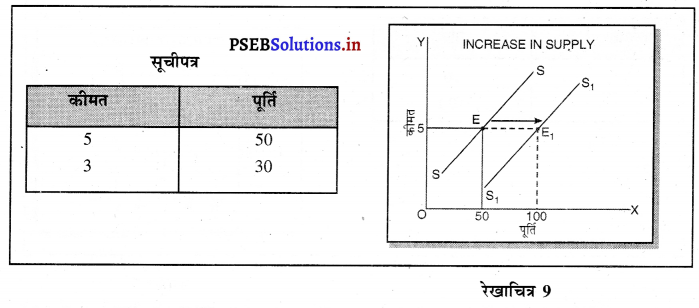
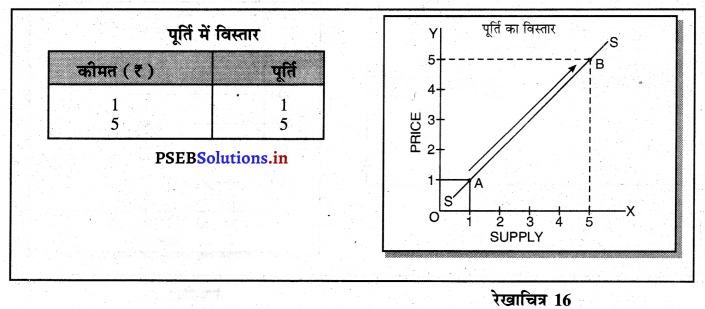
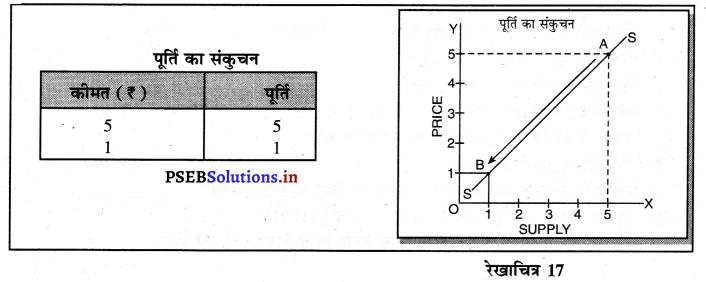
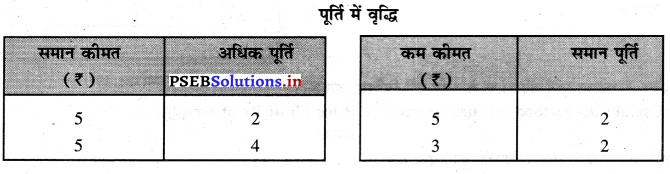
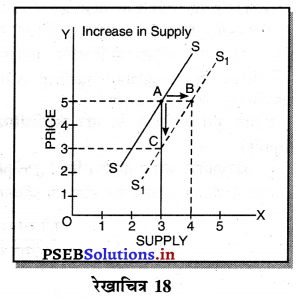
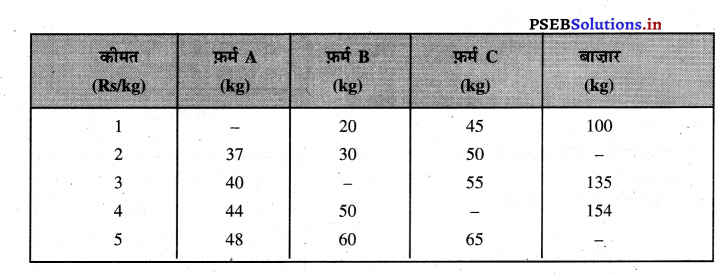
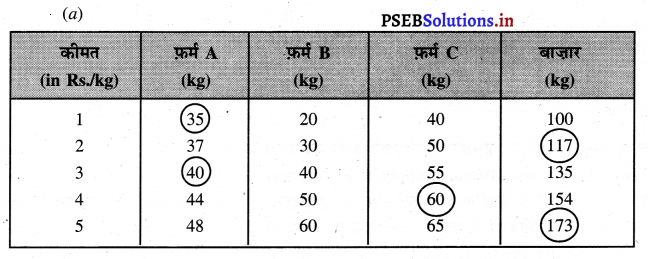
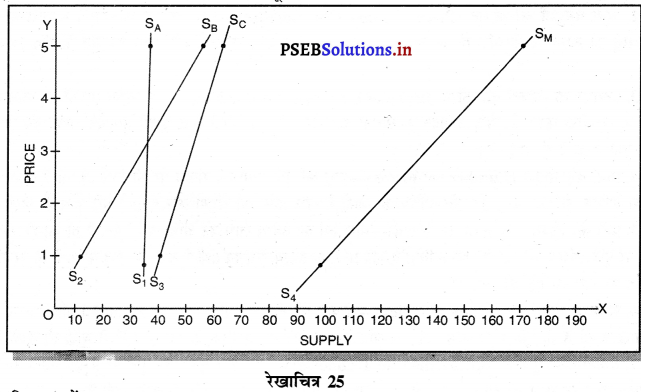
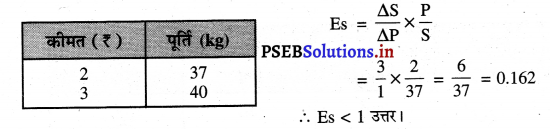
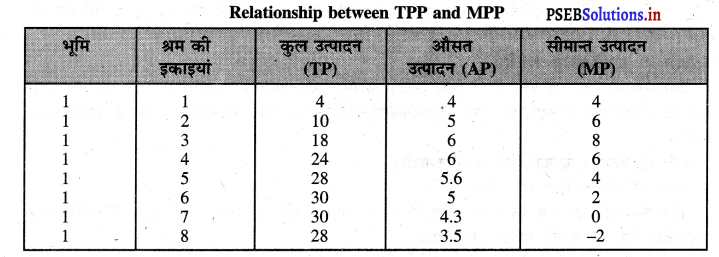
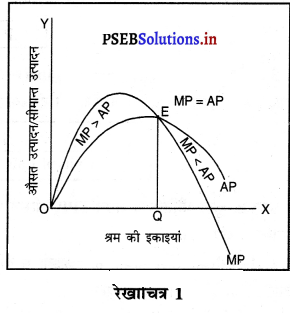
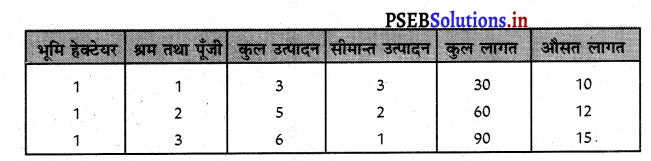
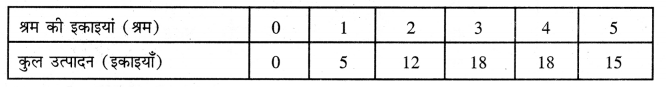
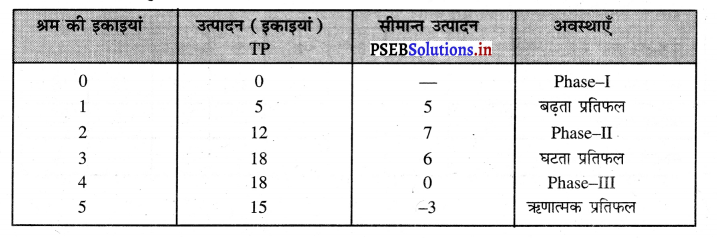
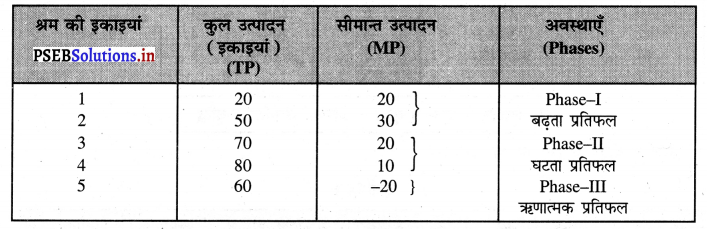
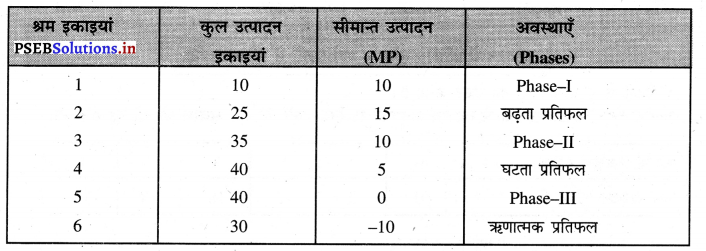
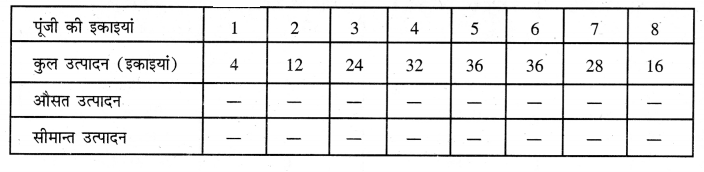
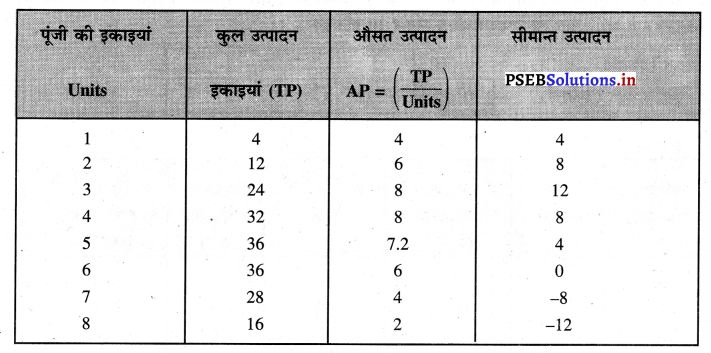
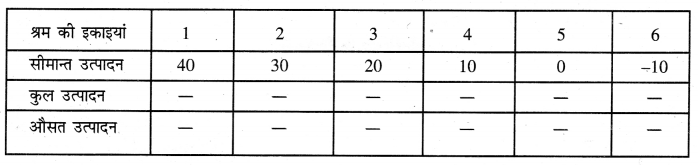
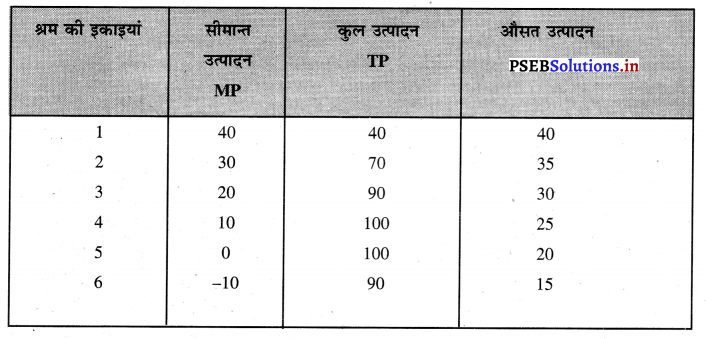
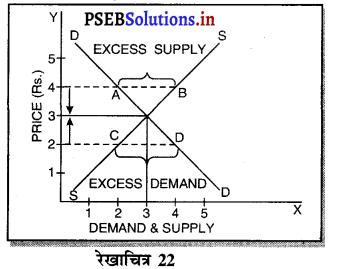
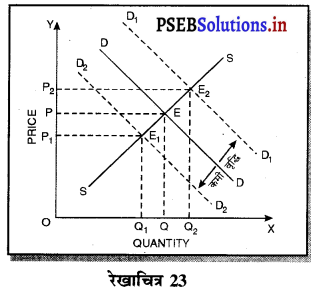
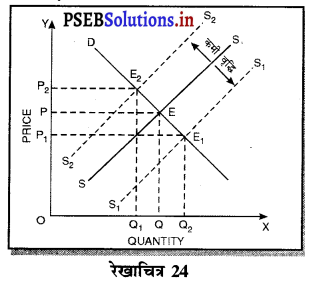
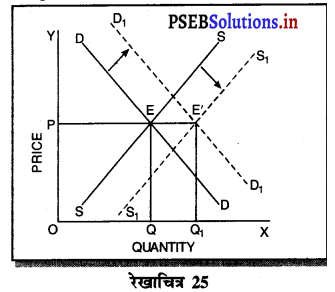
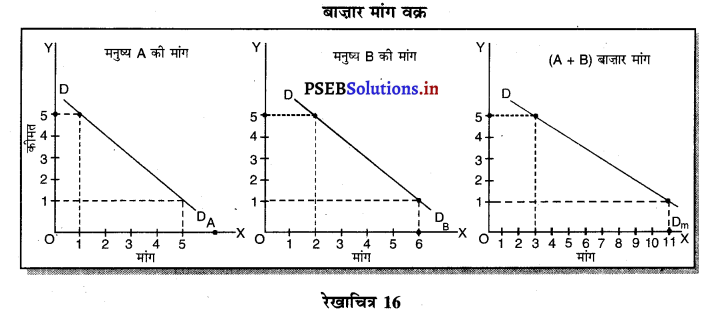
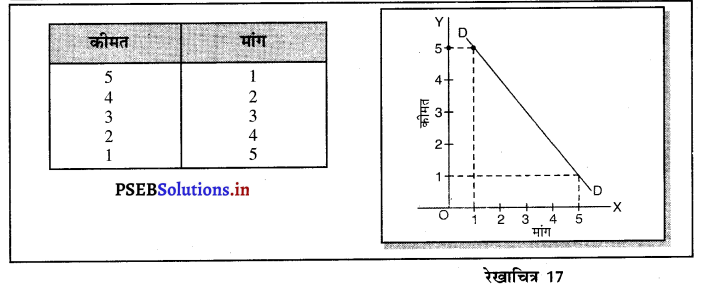
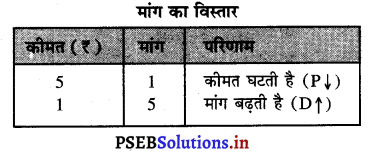
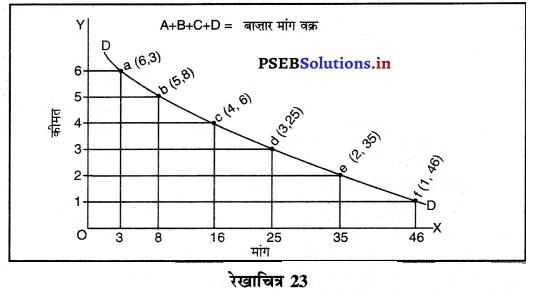
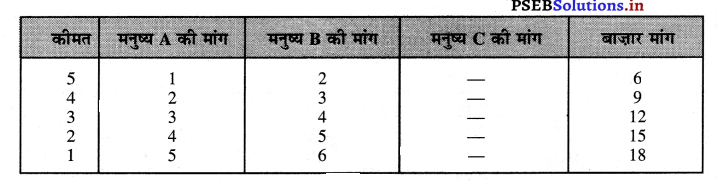
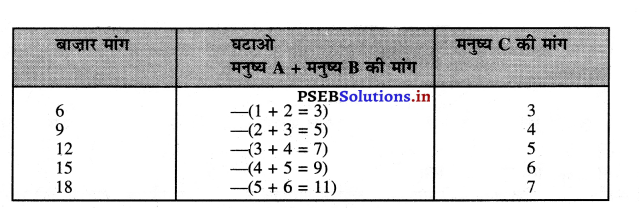
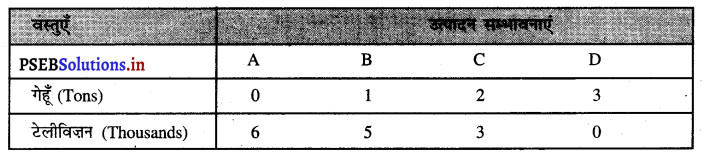
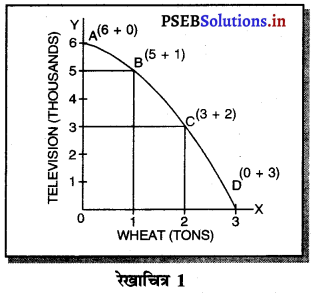
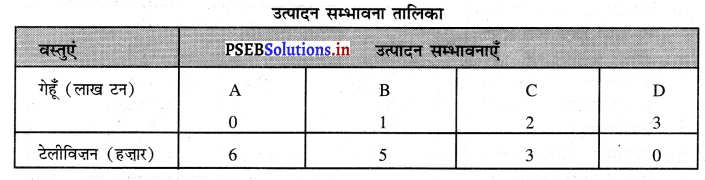
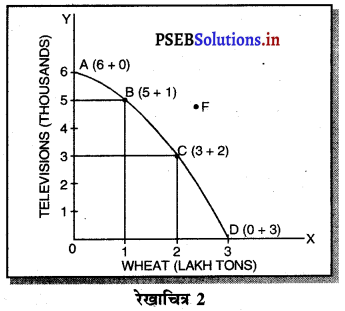
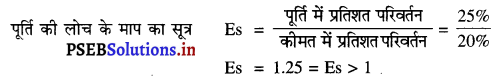Punjab State Board PSEB 11th Class Economics Book Solutions Chapter 12 बाज़ार के रूप Textbook Exercise Questions, and Answers.
PSEB Solutions for Class 11 Economics Chapter 12 बाज़ार के रूप
PSEB 11th Class Economics बाज़ार के रूप Textbook Questions and Answers
I. वस्तुनिष्ठ प्रश्न (Objective Type Questions)
(A) पूर्ण प्रतियोगिता (Perfect Competition)
प्रश्न 1.
बाज़ार से आपका क्या अभिप्राय है ?
उत्तर-
बाज़ार एक ऐसा संयंत्र है जिसमें वस्तु के विक्रेताओं तथा खरीददारों का सुमेल होता है। इसमें किसी वस्तु के बेचने तथा खरीदने के लिए निश्चित भौगोलिक क्षेत्र नहीं होता।
प्रश्न 2.
बाज़ार के विभिन्न रूप बताएँ।
उत्तर-
बाज़ार के प्रमुख रूप हैं-
- पूर्ण प्रतियोगिता
- एकाधिकार
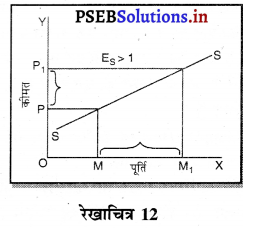
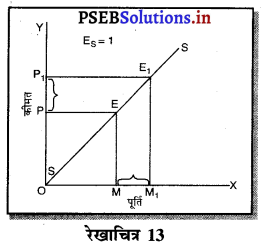
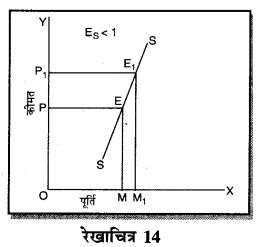
- एकाधिकार प्रतियोगिता।
प्रश्न 3.
पूर्ण प्रतियोगिता की परिभाषा दें।
उत्तर-
पूर्ण प्रतियोगिता वह बाज़ार होता है जहाँ खरीददारों तथा विक्रेताओं की अधिक संख्या होती है। यहाँ पर एक समान वस्तुओं की बिक्री एक कीमत पर की जाती है।
प्रश्न 4.
सम्पूर्ण प्रतियोगिता में किस प्रकार की वस्तु की बिक्री की जाती है ?
उत्तर-
सम्पूर्ण प्रतियोगिता में एक समान वस्तु (Homogenous Product) की बिक्री तथा उत्पादन करते हैं जिनका रंग, रूप, आकार, स्वाद, कीमत, एक-दूसरे से मिलती-जुलती है।
![]()
प्रश्न 5.
पूर्ण प्रतियोगिता में फ़र्म कीमत स्वीकार करने वाली क्यों होती है ?
उत्तर-
बाज़ार का क्रेताओं तथा विक्रेताओं को पूर्ण ज्ञान होता है इसलिए जो कीमत उद्योग में निर्धारित हो जाती है प्रत्येक फ़र्म वह कीमत स्वीकार करती है।
प्रश्न 6.
पूर्ण प्रतियोगिता में क्या असाधारण लाभ तथा हानि सम्भव है ?
उत्तर-
अल्पकाल में कुछ फ़र्मों को असाधारण लाभ तथा हानि हो सकती है।
प्रश्न 7.
पूर्ण प्रतियोगिता में दीर्घ काल में सभी फ़र्मों को कौन-से लाभ होते हैं ?
उत्तर-
पूर्ण प्रतियोगिता में दीर्घकाल में प्रत्येक फ़र्म को साधारण लाभ प्राप्त होते हैं।
प्रश्न 8.
पूर्ण प्रतियोगिता सबसे अधिक व्यावहारिक बाज़ार होता है।
उत्तर-
गलत।
(B) एकाधिकार (Monopoly)
प्रश्न 9.
एकाधिकार से क्या अभिप्राय है ?
उत्तर-
एकाधिकार बाज़ार की वह स्थिति होती है जिसमें वस्तु का उत्पादन इस प्रकार होता है जिसका कोई नज़दीकी स्थानापन्न नहीं होता।
प्रश्न 10.
एकाधिकार बाजार की मुख्य विशेषताएँ बताओ।
उत्तर-
- एक विक्रेता परन्तु बहुत खरीददार
- उत्पादन वस्तु का नज़दीकी स्थानापन्न नहीं होता।
- कीमत पर नियन्त्रण होता है।
- एकाधिकार में कीमत विभेद सम्भव है।
प्रश्न 11.
एकाधिकार फ़र्म की औसत आय तथा सीमान्त आय का आकार कैसा होता है ?
उत्तर-
एकाधिकार बाज़ार में औसत आय तथा सीमान्त आय वक्र की ढलान ऋणात्मक होती है।
![]()
प्रश्न 12.
कीमत विभेद से आपका क्या अभिप्राय है ?
उत्तर-
एक एकाधिकारी अपनी वस्तु की भिन्न ग्राहकों से विभिन्न कीमत प्राप्त करता है तो इसको कीमत विभेद कहते हैं।
प्रश्न 13.
एकाधिकार बाज़ार संरचना का उदय कैसे होता है ?
उत्तर-
एकाधिकार बाज़ार संरचना निम्नलिखित किसी भी कारण से हो सकता है-
- सरकार द्वारा लाइसेंस देना
- पेटेंट अधिकार
- व्यापार गुट
- प्राकृतिक घटना।
प्रश्न 14.
पेटेंट अधिकार से क्या अभिप्राय है ?
उत्तर-
पेटेंट अधिकार का अर्थ है उत्पादित वस्तुओं के नाम, आकार, डिज़ाइन अथवा अन्य विशेषताओं के सम्बन्ध में एकाधिकार का अधिकार प्राप्त करना।
प्रश्न 15.
व्यापारिक गुट से क्या अभिप्राय है ?
उत्तर-
व्यापारिक गुट का अर्थ फ़र्मों के समूह से होता है ताकि सामूहिक निर्णय लेकर, प्रतियोगिता को रोका जा सके और बाज़ार में एकाधिकार स्थापित किया जा सके।
प्रश्न 16.
प्राकृतिक एकाधिकार से क्या अभिप्राय है ?
उत्तर-
एकाधिकार प्राकृतिक घटना भी हो सकती है जब कच्चे माल पर एक उत्पादक का कब्जा हो तो इसे प्राकृतिक एकाधिकार कहा जाता है।
प्रश्न 17.
एकाधिकार में मांग वक्र ऋणात्मक ढाल वाली क्यों होती है ?
उत्तर-
एकाधिकार में माँग वक्र नीचे की ओर झुकी होती है क्योंकि वह कम कीमत पर ही अधिक वस्तु बेच सकता है।
प्रश्न 18.
सामाजिक दृष्टिकोण से एकाधिकार सामाजिक भार Dead weight है। इससे क्या अभिप्राय है?
उत्तर-
इसका अर्थ है कि एकाधिकार समाज के लिए हानिकारक हो सकता है क्योंकि कीमत अधिक निश्चित करके शोषण कर सकता है।
प्रश्न 19.
ट्रस्ट विपक्षीय कानून क्या है ?
उत्तर-
यह कानून फ़र्मों को ट्रस्ट बनाने से रोकते हैं ताकि यह फ़र्फे गुट बनाकर एकाधिकार न बना लें।
(C) एकाधिकारी प्रतियोगिता (Monopolistic Competition)
प्रश्न 20.
एकाधिकार प्रतियोगिता से क्या अभिप्राय है ?
उत्तर-
(Monopoly + Competition = Monopolistic Competition) एकाधिकार प्रतियोगिता में एकाधिकार तथा प्रतियोगिता के गुण पाए जाते हैं।
प्रश्न 21.
एकाधिकार प्रतियोगिता की परिभाषा दें।
उत्तर-
एकाधिकार प्रतियोगिता बाजार का ऐसा रूप है जिसमें क्रेताओं तथा विक्रेताओं की अधिक संख्या होती है तथा इसमें वस्तु विभेद और वस्तु की कीमत पर आंशिक नियन्त्रण पाया जाता है।
![]()
प्रश्न 22.
वस्तु विभिन्नता से आपका क्या अभिप्राय है ?
उत्तर-
वस्तु के रंग, रूप, आकार, गुणवत्ता में अन्तर लाकर उत्पादन करना ही वस्तु विभिन्नता कहलाता है।
प्रश्न 23.
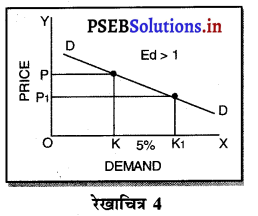
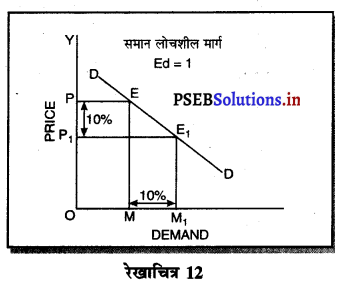
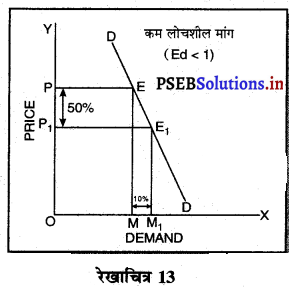
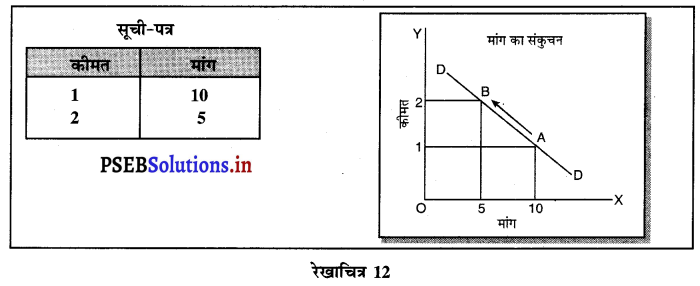
एकाधिकार प्रतियोगिता में माँग वक्र अधिक लोचशील क्यों होती है ?
उत्तर-
एकाधिकार प्रतियोगिता में प्रत्येक उत्पादित वस्तु नज़दीकी स्थानापन्न होती है इसलिए जब एक बेचने वाला वस्तु की कीमत में थोड़ी-सी कमी करता है तो उस वस्तु की माँग बहुत बढ़ जाती है। इसलिए माँग वक्र अधिक लोचशील होती है।
प्रश्न 24.
एकाधिकार और एकाधिकार प्रतियोगिता में कौन-सी दो समानताएँ होती हैं ?
उत्तर-
- AR तथा MR ऋणात्मक ढाल वाली होती है।
- दोनों बाज़ारों में कीमत पर पूर्ण तथा आंशिक नियन्त्रण होता है।
प्रश्न 25.
पूर्ण प्रतियोगिता और एकाधिकार प्रतियोगिता में कौन-सी दो समानताएँ होती हैं ?
उत्तर-
- दोनों बाजारों में खरीददार और बेचने वालों की संख्या अधिक होती है।
- दोनों बाज़ारों में दीर्घकाल में साधारण लाभ प्राप्त होता है।
प्रश्न 26.
बिक्री लागत अथवा प्रचार लागत अथवा प्रेरणा प्रचार से क्या अभिप्राय है ?
उत्तर-
एकाधिकार प्रतियोगिता में वस्तु की बिक्री बढ़ाने के लिए बिक्री लागतें अथवा प्रचार लागतें व्यय की जाती हैं जिससे लाभ में वृद्धि होती है।
प्रश्न 27.
एकाधिकार प्रतियोगिता के बाजार में कीमत तथा सीमान्त लागत का क्या सम्बन्ध हैं ?
उत्तर-
एकाधिकारी प्रतियोगिता के बाज़ार में सन्तुलन MR = MC द्वारा स्थापित होता है परन्तु इसमें कीमत, औसत लागत से अधिक होती है (P > HC)
प्रश्न 28.
अपूर्ण बाज़ार (Imperfect-Competition) के तीन रूप कौन-कौन से हैं ?
उत्तर-
- एकाधिकारी प्रतियोगिता (Monopolistic Competition)
- अल्पाधिकार (Oligopoly)
- दोहरा अधिकार (Duopoly)
प्रश्न 29.
अल्पाधिकार (Oligopoly) से क्या अभिप्राय है ?
उत्तर-
जिस बाज़ार में 2 से 8 तक उत्पादक होते हैं उस बाज़ार को अल्पाधिकार का बाज़ार कहा जाता है।
प्रश्न 30.
दोहरा अधिकार (Duopoly) से क्या अभिप्राय है ?
उत्तर-
जब एक बाज़ार में दो उत्पादक होते हैं तो इसको दोहरा अधिकार का बाज़ार कहा जाता है।
प्रश्न 31.
एकाधिकार प्रतियोगिता के बाज़ार की दो उदाहरणे दें।
उत्तर-
- टूथपेस्ट बाज़ार
- टेलीविज़न बाज़ार।
प्रश्न 32.
पूर्ण प्रतियोगिता में वस्तुएँ ……. होती हैं।
(a) समरूप
(b) विषमांगी
(c) वस्तु विभिन्नता
(d) उपरोक्त सभी।
उत्तर-
(a) समरूप।
प्रश्न 33.
एकाधिकार में वस्तुएँ ………. होती हैं।
(a) समरूप
(b) लासानी
(c) विषमांगी
(d) इन में से कोई नहीं।
उत्तर-
(b) लासानी।
![]()
प्रश्न 34.
एकाधिकार प्रतियोगिता में वस्तुएँ ……….. होती है।
(a) समरूप
(b) लासानी
(c) वस्तु विभिन्नता
(d) उपरोक्त सभी।
उत्तर-
(c) वस्तु विभिन्नता।
प्रश्न 35.
पर्ण प्रतियोगिता में फ़र्म कीमत …………. करती है।
(a) निर्धारण
(b) स्वीकार
(c) निर्धारण तथा स्वीकार
(d) ननिर्धारण न स्वीकार
उत्तर-
(a) स्वीकार।
प्रश्न 36.
पूर्ण प्रतियोगिता में फ़र्म की माँग वक्र …….. होती है।
(a) पूर्ण लचकदार
(b) पूर्ण बेलोचदार
(c) अधिक लचकदार
(d) इनमें से कोई भी नहीं।
उत्तर-
(a) पूर्ण लचकदार।
प्रश्न 37.
पूर्ण प्रतियोगिता और शुद्ध प्रतियोगिता में अन्तर ………. का होता है।
उत्तर-
डिग्री।
प्रश्न 38.
एकाधिकार बाजार में उत्पादक इस प्रकार की वस्तु का उत्पादन करता है जिसका ………… नहीं होता।
उत्तर-
निकट स्थानापन्न।
प्रश्न 39.
एकाधिकार प्रतियोगिता का मुख्य लक्षण …………… होता है।
(a) समरूप वस्तुएँ
(b) लासानी वस्तुएँ
(c) वस्तु विभिन्नता
(d) उपरोक्त सभी।
उत्तर-
(c) वस्तु विभिन्नता।
प्रश्न 40.
एकाधिकार में उत्पादक वस्तु की कीमत को ……… करता है।
(a) निर्धारित
(b) स्वीकार
(c) निर्धारित और स्वीकार
(d) निर्धारित और न ही स्वीकार।
उत्तर-
(a) निर्धारित।
प्रश्न 41.
एकाधिकार प्रतियोगिता में प्रचार पर व्यय को ………. कहते हैं।
उत्तर-
बिक्री लागतें।
प्रश्न 42.
उत्पादक के सन्तुलन की शर्ते बताएँ।
उत्तर-
- MR = MC.
- MC वक्र MR रेखा को नीचे से ऊपर की तरफ जाती हुई काटे।
प्रश्न 43.
उत्पादक के सन्तुलन की पहली शर्त ………. होती है और दूसरी शर्ते MC वक्र MR को नीचे से ऊपर को जाती हुई काटे।
उत्तर-
MR = MC.
प्रश्न 44.
सामाजिक दृष्टिकोण से एकाधिकार सामाजिक भार (Dead Weight) है।
उत्तर-
सही।
प्रश्न 45.
एकाधिकार प्रतियोगिता व्यावहारिक बाज़ार की स्थिति होती है।
उत्तर-
सही।
प्रश्न 46.
एकाधिकार प्रतियोगिता में प्रचार पर किये गए व्यय को ….. लागतें कहा जाता है।
उत्तर-
बिक्री।
![]()
प्रश्न 47.
पूर्ण प्रतियोगिता में औसत आय और सीमान्त आय ……… लोचदार रेखाएँ होती हैं।
उत्तर-
आंशिक।
प्रश्न 48.
विभिन्न ग्राहकों को एक वस्तु अलग-अलग कीमतों पर बेचने को ……. का बाज़ार कहते हैं।
उत्तर-
कीमत विभेद।
प्रश्न 49.
एकाधिकार और एकाधिकारी प्रतियोगिता में औसत आय तथा सीमान्त आय ……. ढलान वाली रेखाएं होती हैं।
उत्तर-
ऋणात्मक।
प्रश्न 50.
एकाधिकार में वस्तु ….. होती है।
(a) समरूप
(b) विलक्षण
(c) विषमरूप
(d) कोई भी नहीं।
उत्तर-
(b) विलक्षण।
प्रश्न 51.
एक व्यवस्था जिसमें केवल दो उत्पादक ही होते हैं को ………. कहते हैं।
(a) एकाधिकार
(b) दो-अधिकार
(c) अल्पाधिकार
(d) कोई भी नहीं।
उत्तर-
(b) दो-अधिकार।
प्रश्न 52.
अपूर्ण प्रतियोगिता में वस्तुएँ ……… होती हैं।
(a) समरूप
(b) विलक्षण
(c) विषम अंगी
(d) कोई भी नहीं।
उत्तर-
(c) विषम अंगी।
प्रश्न 53.
अल्प-अधिकार बाज़ार 2 से 10 तक उत्पादक होते हैं।
उत्तर-
सही।
प्रश्न 54.
जिस बाज़ार में दो उत्पादक होते हैं, उसको …….. कहा जाता है।
उत्तर–
दोहरा अधिकार (Duopoly) ।
प्रश्न 55.
दोहरा-अधिकार बाजार अल्प-अधिकार का सरल रूप है।
उत्तर-
सही।
प्रश्न 56.
अल्प-अधिकार बाज़ार में बिक्री लागतें (Selling Costs) नहीं होती।
उत्तर-
गलत।
प्रश्न 57.
अल्प-अधिकार में मांग वक्र ………… होती है।
(a) पूर्ण लोचदार
(b) पूर्ण बेलोचदार
(c) अनिश्चित
(d) कोई भी नहीं।
उत्तर-
(c) अनिश्चित।
प्रश्न 58.
अल्प बाज़ार एक व्यावहारिक बाज़ार है।
उत्तर-
सही।
II. अति लघु उत्तरीय प्रश्न (Very Short Answer Type Questions)
(A) पूर्ण प्रतियोगिता (Perfect Competition)
प्रश्न 1.
बाजार से आपका क्या अभिप्राय है ? बाजार के विभिन्न रूप बताओ।
उत्तर-
बाज़ार एक ऐसी प्रक्रिया है जिसमें वस्तु के विक्रेताओं तथा खरीददारों को एकत्रित किया जाता है। यह कोई निश्चित भौगोलिक क्षेत्र नहीं होता। बाज़ार के विभिन्न रूप हैं-
- पूर्ण प्रतियोगिता
- एकाधिकार
- एकाधिकारी प्रतियोगिता
- अल्पाधिकार (oligopoly)।
अल्पाधिकार में बड़े आकार के थोड़े से बेचने वाले होते हैं, जैसे पैप्सी कोला तथा कोका कोला।
प्रश्न 2.
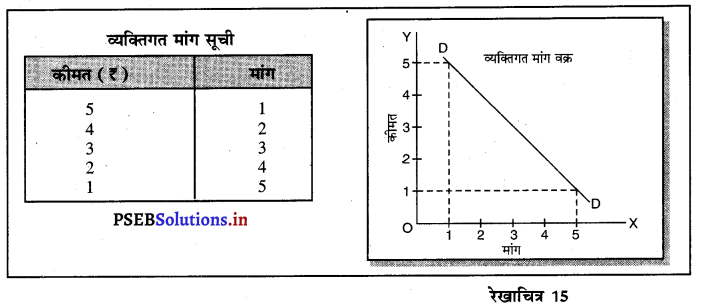
पूर्ण प्रतियोगिता की दो मुख्य विशेषताएं बताओ।
उत्तर-
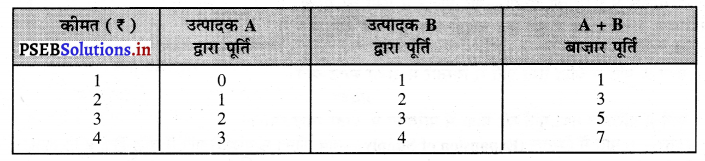
- खरीददारों तथा बेचने वालों की बड़ी संख्या- इस बाज़ार में बेचने तथा खरीदने वालों की बड़ी संख्या होती है। कोई एक बेचने अथवा खरीदने वाला वस्तु की कीमत को प्रभावित नहीं कर सकता।
- एक समान वस्तुएं-प्रत्येक उत्पादक द्वारा उत्पादन की वस्तुएं (Homogenous) होती हैं जिनका रंग, रूप, आकार, स्वाद तथा कीमत, एक दूसरे से मिलती-जुलती होती हैं।
प्रश्न 3.
सम्पूर्ण प्रतियोगिता में एक फ़र्म कीमत स्वीकार करने वाली क्यों होती है ?
उत्तर-
- सम्पूर्ण प्रतियोगिता में विक्रेता एक समान वस्तु (Homogenous Product) की बिक्री करते हैं। इसलिए अलग कीमत प्राप्त करनी सम्भव नहीं होती।।
- सम्पूर्ण प्रतियोगिता में विक्रेताओं की संख्या बहुत अधिक होती है। यदि फ़र्म अधिक कीमत प्राप्त करने का प्रयत्न करती है तो मांग बहुत कम हो जाएगी तथा यदि कीमत प्राप्त करती है तो मांग इतनी बढ़ जाएगी, जिसको पूरा करना सम्भव नहीं होगा तथा उस फ़र्म को हानि होगी। इसलिए फ़र्म कीमत स्वीकार करने वाली होती है।
प्रश्न 4.
पूर्ण प्रतियोगिता में दीर्घकाल में फ़र्म का सन्तुलन किस स्थिति में होता है ?
अथवा
दीर्घकाल में पूर्ण प्रतियोगिता फ़र्म अधिकतम लाभ कब प्राप्त करती है ?
उत्तर-
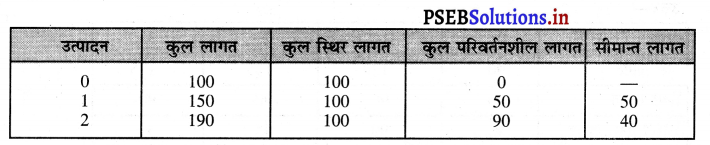
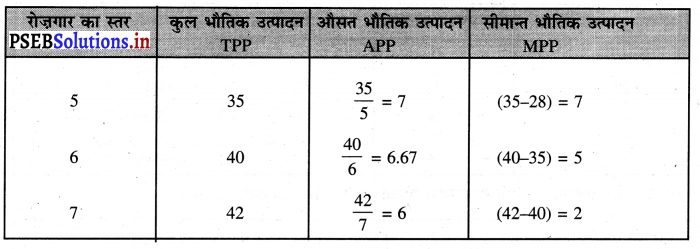
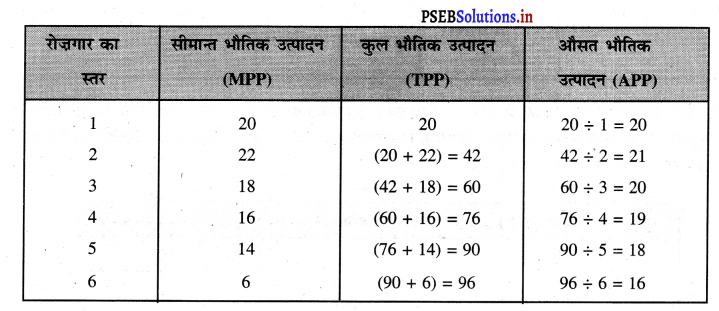
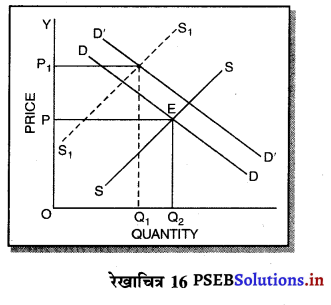
पूर्ण प्रतियोगिता में तथा दीर्घकाल में सभी फ़र्मों को असाधारण लाभ शून्य (zero abnormal profits) प्राप्त होते हैं। प्रत्येक फ़र्म केवल साधारण लाभ प्राप्त करती है। फ़र्म का सन्तुलन न उस स्थिति में होता है। जहां उसको अधिकतम लाभ प्राप्त होता है। MR = LMC and P = LAC OR P = LMC = LAC
![]()
प्रश्न 5.
पूर्ण प्रतियोगिता में एक समान वस्तुओं से क्या अभिप्राय है ? इसका बाज़ार में कीमत पर क्या प्रभाव पड़ता है ?
उत्तर–
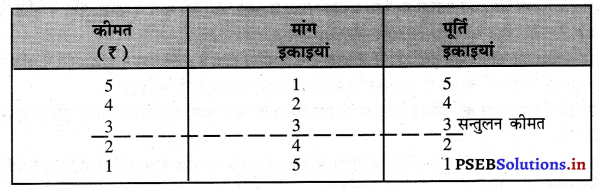
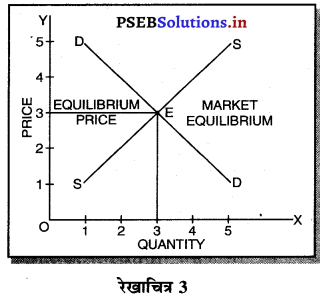
पूर्ण प्रतियोगिता में प्रत्येक उत्पादक एक समान (Homogenous Product) का उत्पादन करता है। एकसमान वस्तुओं का अर्थ है कि वस्तु का रंग, रूप, आकार, स्वाद, गुणवता इत्यादि एक-दूसरे से मिलती जुलती होती हैं। इससे बाज़ार में उस वस्तु की एक कीमत (one price) निश्चित हो जाती है।
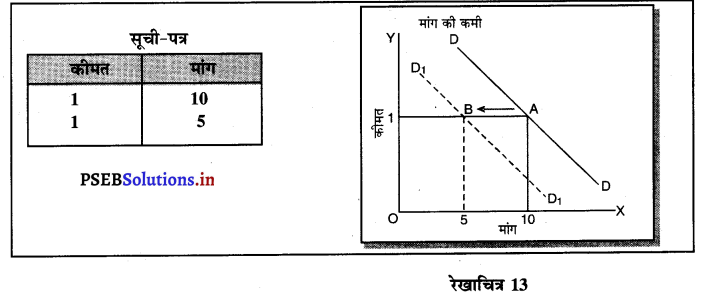
प्रश्न 6.
पूर्ण प्रतियोगिता में मांग वक्र पूर्ण लोचशील क्यों होती है ?
उत्तर-
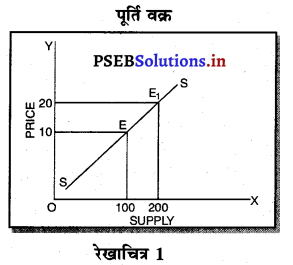
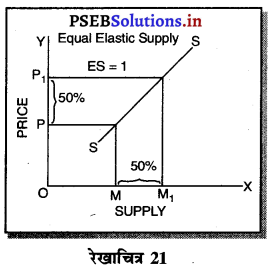
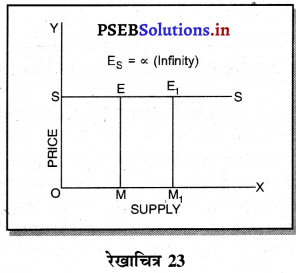
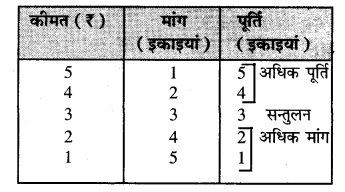
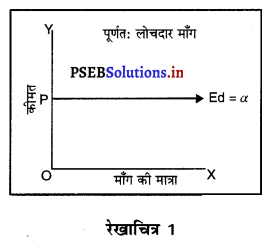
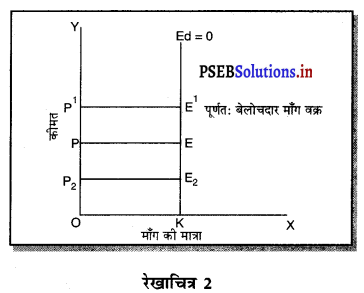
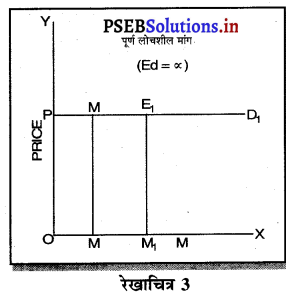
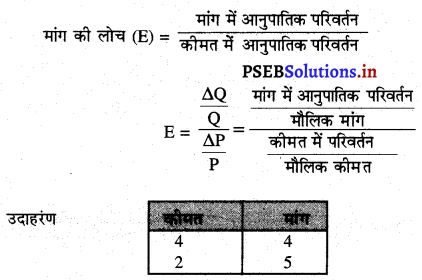
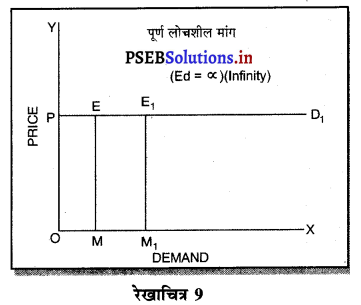
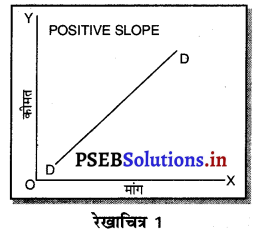
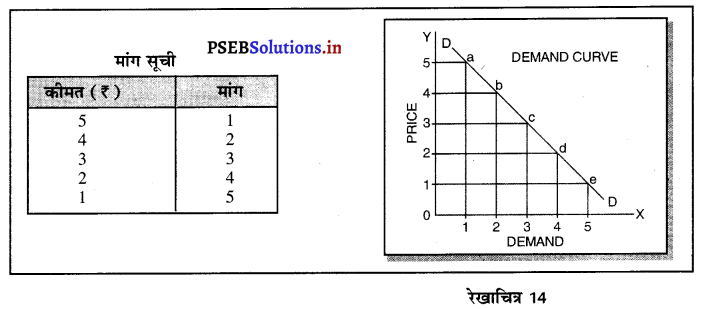
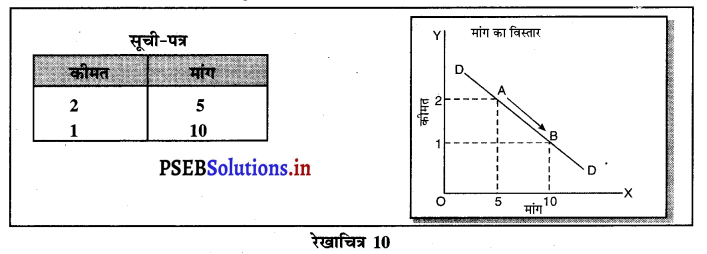
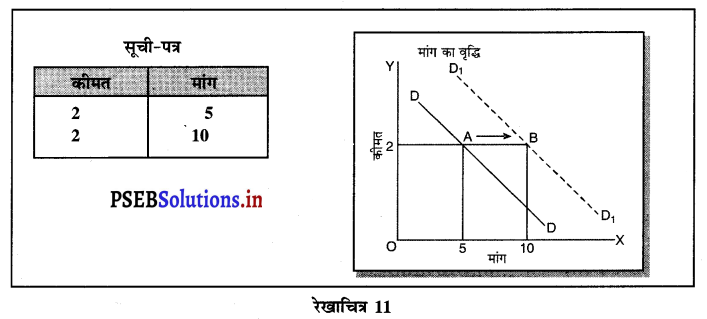
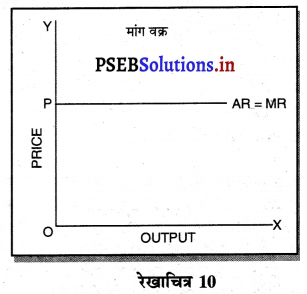
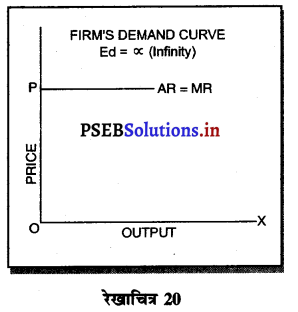
पूर्ण प्रतियोगिता में मांग वक्र पूर्ण लोचशील होती है, जोकि Ox के समान्तर बनती है। इसमें मांग की लोच पूर्ण लोचशील (Ed = ∝) होती है। यदि कोई फ़र्म बाज़ार कीमत से थोड़ी अधिक कीमत लेती है, तो उसकी मांग शून्य हो जाएगी। यदि कम कीमत लेती है तो मांग अनन्त तक बढ़ जाती है। इस कारण मांग वक्र पूर्ण लोचशील होती है।
प्रश्न 7.
पूर्ण प्रतियोगिता में यदि कुछ फ़र्मों को असाधारण लाभ होता है तो फ़र्मों की संख्या पर क्या प्रभाव पड़ता है ? यदि असाधारण हानि होती है तो फ़र्मों की संख्या पर क्या प्रभाव पड़ता है ?
उत्तर-
- पूर्ण प्रतियोगिता में यदि कुछ फ़र्मों को असाधारण लाभ होता है तो इस उद्योग में दीर्घकाल में नई फ़र्मे प्रवेश कर जाती हैं।
- यदि कुछ फ़र्मों को हानि होती है तो दीर्घकाल में जिन फ़र्मों को हानि होती है, वह उद्योग को छोड़ जाती हैं।
(B) एकाधिकार (Monopoly)
प्रश्न 8.
एकाधिकार से क्या अभिप्राय है ?
उत्तर-
एकाधिकार, बाजार की वह स्थिति होती है, जिसमें वस्तु का उत्पादक एक होता है तथा उस वस्तु का कोई नज़दीकी स्थानापन्न (Close substitute) नहीं होता। इसलिए एकाधिकार वह स्थिति है, जिसमें उत्पादन पर केवल एक फ़र्म का नियन्त्रण हो। फ़र्म तथा उद्योग में कोई अन्तर नहीं होता।
प्रश्न 9.
व्यापारिक गुट से आपका क्या अभिप्राय है ? जब दो फ़र्मे मिल जाती हैं तो इससे कार्य कुशलता में वृद्धि कैसे होती है ?
उत्तर-
जब फ़र्फे स्वतन्त्र तौर पर कार्य करती हैं परन्तु उत्पादन तथा कीमत सम्बन्धी समझौता कर लेती हैं तो इसको व्यापारिक गुट कहा जाता है। मान लो एक उद्योग में दो फ़मैं हैं, दोनों की कार्यकुशलता कम है। एक फ़र्म के पास तकनीकी माहिर श्रमिक हैं, परन्तु अच्छे मण्डीकरण की जांच नहीं है। दूसरी फ़र्म के पास मण्डीकरण की अच्छी महारत है, परन्तु तकनीकी माहिर श्रमिक नहीं हैं। यदि यह फ़मैं एकत्रित होकर एकाधिकार स्थापित कर लें तो दोनों फ़र्मों के मिल जाने से दोनों की कार्य कुशलता बढ़ जाएगी।
प्रश्न 10.
एकाधिकार में कुल आय वक्र का क्या आकार होता है ?
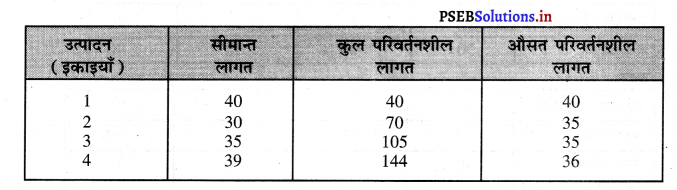
उत्तर-
एकाधिकार में कुल आय आरम्भ में घटती दर पर बढ़ती है। जब सीमान्त आय घटती है। कुल आय अधिकतम होती है, जब सीमान्त आय शून्य हो जाती है। कुल आय घटती है, जब सीमान्त आय ऋणात्मक हो जाती है। इस प्रकार कुल आय वक्र का आकार उलटे U जैसा होता है।
प्रश्न 11.
एकाधिकार में औसत आय तथा सीमान्त आय वक्र का आकार किस प्रकार का होता है ?
उत्तर-
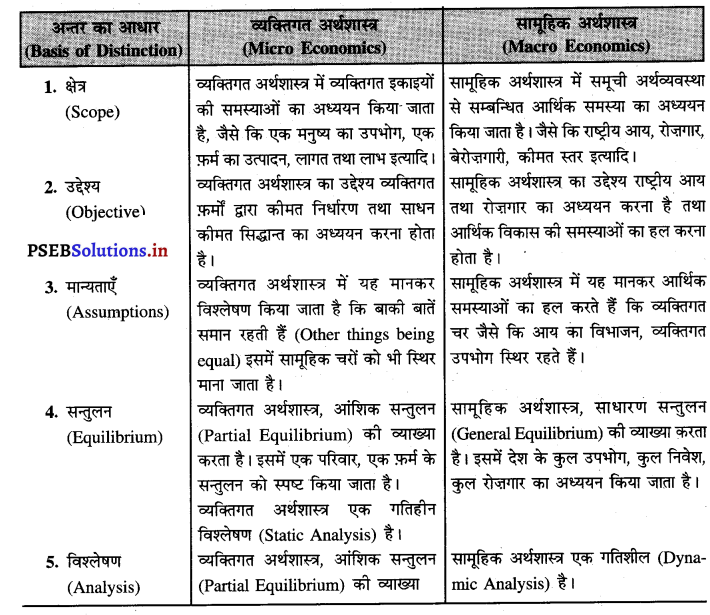
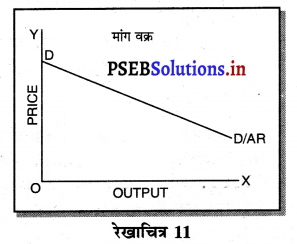
एकाधिकार में औसत आय को कीमत वक्र भी कहा जाता है। कीमत में कमी करके ही मांग में वृद्धि की जा सकती है। इसलिए औसत आय AR नीचे को घटती रेखा होती है जब AR घटती है तो MR तीव्रता से घटती है। इस प्रकार औसत आय तथा सीमान्त आय का ऋणात्मक आकार होता है।
प्रश्न 12.
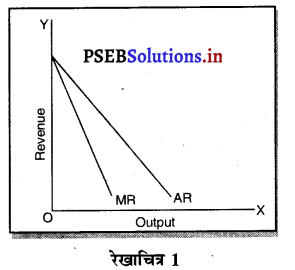
एकाधिकारी सन्तुलन में उत्पादन तथा MC कीमत पूर्ण प्रतियोगिता में सन्तुलन उत्पादन तथा कीमत से तुलनात्मक कैसे होती है ?
उत्तर-
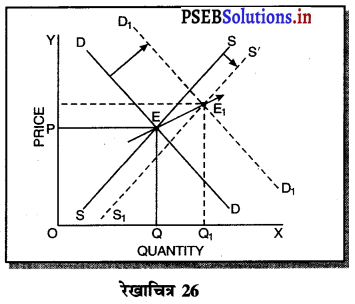
- एकाधिकारी में सन्तुलन AR तथा MR घटती रेखाओं से सन्तुलन E बिन्दु पर होता है। इससे कीमत Pm तथा उत्पादन Qm होता है।
- पूर्ण प्रतियोगिता में AR = MR सीधी रेखा पर सन्तुलन E1 पर होता है तथा कीमत Pp तथा उत्पादन Qp होता है। स्पष्ट है कि एकाधिकारी में कीमत अधिक तथा उत्पादन कम किया जाता है।
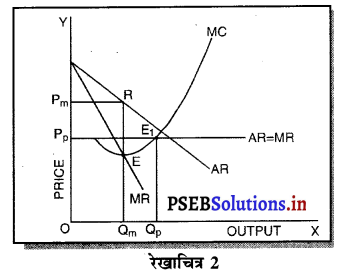
प्रश्न 13.
कीमत विभेद से आपका क्या अभिप्राय है ? कीमत विभेद कौन-से बाज़ार में सम्भव होता है ?
उत्तर-
जब एक एकाधिकारी अपनी वस्तु की विभिन्न ग्राहकों से विभिन्न कीमत प्राप्त करता है ताकि अधिक लाभ प्राप्त कर सके तो इसको कीमत विभेद कहा जाता है। कीमत विभेद केवल एकाधिकार बाजार में ही सम्भव होता है। उदाहरणस्वरूप एक डॉक्टर अमीर लोगों से दवाई की कीमत के साथ मश्वरा फीस प्राप्त करता है तथा निर्धनों से दवाई के पैसे ही लेता है अथवा पुरुषों से स्त्रियों को आय कर कम कर देना पड़ता है।
![]()
प्रश्न 14.
पेटेंट अधिकार क्या है ? पेटेंट अधिकारों का क्या उद्देश्य होता है ?
उत्तर-
जब कोई फ़र्म नई वस्तु अथवा तकनीक की खोज के पश्चात् पेटेंट अधिकार प्राप्त करती है तो कोई अन्य फ़र्म उस वस्तु अथवा तकनीक का प्रयोग नहीं कर सकती। इसका उद्देश्य यह होता है कि अन्य फ़मैं नई खोजें करने तथा नई खोजों से वस्तु का उत्पादन किया जाए ताकि खोज के कार्य का विकास हो। कितने वर्ष के लिए पेटेंट अधिकार दिया जाता है, उसको पेटेंट जीवन (Patent life) कहा जाता है।
प्रश्न 15.
ट्रस्ट विपक्षीय कानून क्या है ?
उत्तर-
ट्रस्ट विपक्षीय कानून (Anti Trust Legistation)-वह कानून है जोकि बड़ी फ़र्मों को ट्रस्ट बनाने से रोकते हैं ताकि यह फ़र्मे व्यापारिक गुट बनाकर एकाधिकार न बना लें। एकाधिकार बनाने से बड़ी फ़र्मे लोगों का शोषण करने लगती है। इसलिए ट्रस्ट विपक्षीय कानून बनाए जाते हैं।
प्रश्न 16.
एकाधिकारी फ़र्म की सीमान्त आय (MR) औसत आय (AR) से कम क्यों होती है ?
उत्तर-
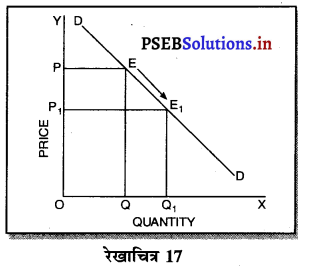
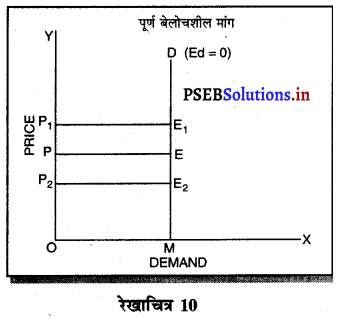
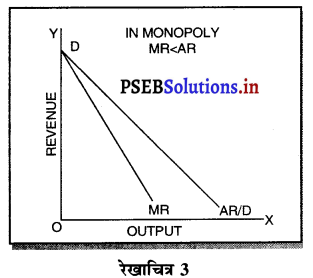
एकाधिकार फ़र्मे की सीमान्त आय (MR) औसत आय (AR) से कम होती है, क्योंकि एकाधिकार फ़र्म वस्तु की कीमत (AR) में कमी करके ही अधिक वस्तुओं की बिक्री कर सकती है। जब कीमत अथवा औसत आय घटाई जाती है तो सीमान्त आय में कमी तीव्रता से होती है। इस कारण एकाधिकार में मांग वक्र DD ऋणात्मक ढलान वाली होती है।
(C) एकाधिकारी प्रतियोगिता (Monopolistic Competition)
प्रश्न 17.
एकाधिकारी प्रतियोगिता से क्या अभिप्राय है ?
उत्तर-
एकाधिकारी प्रतियोगिता में खरीददार तथा बेचने वाले बहुत होते हैं। प्रत्येक उत्पादक वस्तु भिन्नता द्वारा अपनी वस्तु को विभिन्न बनाने का प्रयत्न करता है। वस्तु की कीमत पर फ़र्म का आंशिक नियन्त्रण होता है। प्रचार लागतों द्वारा वस्तु अधिक बेचने का प्रयत्न किया जाता है। आधुनिक बाजार को एकाधिकारी प्रतियोगिता का बाज़ार कहा जाता है।
प्रश्न 18.
वस्तु भिन्नता से आपका क्या अभिप्राय है ?
उत्तर-
एकाधिकारी प्रतियोगिता में प्रत्येक उत्पादक अपनी वस्तु को विभिन्न बनाने के प्रयत्न करता है। वस्तु के रंग, रूप, आकार, भार, गुणवत्ता में अन्तर लाकर ग्राहकों को खींचने का प्रयत्न किया जाता है। जब उत्पादक वस्तुओं के उत्पादन में थोड़ा अन्तर करते हैं तो इसको वस्तु भिन्नता कहा जाता है।
प्रश्न 19.
बिक्री लागतों से क्या अभिप्राय है ?
अथवा
प्रचार लागतें क्या होती हैं ?
अथवा
प्रेरणा प्रचार से क्या अभिप्राय है ?
उत्तर-
एकाधिकारी प्रतियोगिता में प्रत्येक फ़र्म वस्तु की बिक्री बढ़ाने के लिए बिक्री लागतों अथवा प्रचार लागतों पर व्यय करती है। प्रचार द्वारा लोगों को वस्तु के गुणों की जानकारी दी जाती है ताकि इससे प्रेरणा लेकर लोग वस्तु की अधिक मात्रा की खरीद करें।
प्रश्न 20.
बाज़ार की मुख्य विशेषताएं बताइए।
उत्तर-
बाज़ार की मुख्य विशेषताएं इस प्रकार हैं –
- क्षेत्र-वह सारा क्षेत्र जहां वस्तुएं बेची अथवा खरीदी जाती हैं।
- क्रेता और विक्रेता-किसी बाजार के लिए खरीदने तथा बेचने वालों का होना अति आवश्यक है।
- सम्पर्क-क्रेता और विक्रेता में परस्पर व्यापारिक सम्बन्ध होना ज़रूरी है।
- एक वस्तु-अर्थशास्त्र में हर एक वस्तु का बाज़ार अलग-अलग होता है।
- प्रतियोगिता-क्रेता तथा विक्रेता में प्रतियोगिता पाई जाती है।
- एक कीमत-सारे बाज़ार में एक प्रकार की वस्तु की एक कीमत निश्चित हो जाती है।
(D) अल्प-अधिकार (Oligolopy)
प्रश्न 21.
अल्प अधिकार से क्या अभिप्राय है ? ।
उत्तर-
अल्प अधिकार एक व्यावहारिक बाज़ार है जो कि वास्तविक नज़र आता है। इस बाज़ार में विक्रेताओं की संख्या 2 से 10 तक होती है। यह उत्पादक एक दूसरे के नज़दीकी स्थानापन्न उत्पाद बनाते हैं। प्रत्येक उत्पादक वस्तु से भिन्नता करके उत्पाद को अलग बनाने का यत्न करता है।
प्रश्न 22.
अल्प-अधिकार की कोई दो विशेषताएं बताएं।
उत्तर-
अल्प-अधिकार बाज़ार की दो विशेषताएं इस प्रकार हैं –
- अन्तर्निर्भरता-अल्प-अधिकार बाज़ार में फर्मे एक-दूसरे पर निर्भर होती हैं। इस बाज़ार में जिन वस्तुओं का उत्पादन किया जाता है वह एक-दूसरे के स्थानापन्न होते हैं। जैसे टी०वी० वाशिंग मशीन आदि एक उत्पादक द्वारा लिया गया निर्णय दूसरे उत्पादकों को प्रभावित करता है। यदि एक उत्पादक कीमत में कमी करता है तो दूसरे उत्पादकों को भी कमी करनी पड़ती है।
- प्रचार-इस बाज़ार पर बहुत व्यय किया जाता है। प्रचार करके प्रत्येक उत्पादक अधिक बिक्री करने का यत्न करता है।
III. लघु उत्तरीय प्रश्न (Short Answer Type Questions)
A. पूर्ण प्रतियोगिता (Perfect Competition)
प्रश्न 1.
बाज़ार से क्या अभिप्राय है ? इसकी विशेषताओं का वर्णन करें।
उत्तर-
अर्थशास्त्र में बाज़ार का अर्थ आम भाषा से अलग लिया जाता है। अर्थशास्त्र में बाज़ार का अर्थ विशेष स्थान से नहीं होता बल्कि उस क्षेत्र से होता है जहां तक एक वस्तु की एक कीमत होने की प्रवृत्ति होती है। क्रेताओं तथा विक्रेताओं में एक दूसरे से सम्पर्क टैलीफोन, तार, एजैन्ट द्वारा स्थापित किया जा सकता है उनका प्रत्यक्ष सम्पर्क ज़रूरी नहीं होता। विशेषताएं (Characteristics)-
- बाज़ार से अभिप्राय उस क्षेत्र से होता है जहां पर खरीददार और विक्रेता फैले होते हैं।
- क्रेताओं और विक्रेताओं से प्रत्यक्ष सम्बन्ध ज़रूरी नहीं बल्कि किसी माध्यम से हो सकता है।
- अर्थशास्त्र में प्रत्येक वस्तु का भिन्न बाज़ार होता है। जैसा कि गेहूं का बाज़ार, चीनी का बाज़ार इत्यादि।
- क्रंताओं तथा विक्रेताओं में प्रतियोगिता पाई जाती है।
प्रश्न 2.
पूर्ण प्रतियोगिता की कोई चार विशेषताएं बताओ।
उत्तर-
पूर्ण प्रतियोगिता की मुख्य चार विशेषताएं निम्नलिखित हैं-
- खरीदने तथा बेचने वालों की बड़ी संख्या-पूर्ण प्रतियोगिता में खरीदने तथा बेचने वालों की संख्या इतनी अधिक होती है कि कोई एक खरीददार अथवा विक्रेता वस्तु की कीमत को प्रभावित नहीं कर सकता।
- एकसमान वस्तुएं-सभी उत्पादक एक समान वस्तुओं का उत्पादन करते हैं अर्थात् प्रत्येक उत्पादक की वस्तु का रंग रूप, आकार, स्वाद, एक दूसरे से मिलता जुलता होता है।
- प्रवेश तथा छोड़ने की स्वतन्त्रता-कोई भी फ़र्म अथवा उत्पादक किसी उद्योग में जब मर्जी शामिल हो सकता है तथा जब चाहे उस उद्योग को छोड़ सकता है। प्रवेश करने अथवा छोड़ते समय किसी से आज्ञा नहीं लेनी पड़ती।
- एक कीमत-इस बाज़ार में एक समय एक वस्तु की एक कीमत निर्धारण हो जाती है। इस कीमत पर एक फ़र्म जितनी चाहें वस्तुएं बेच सकती हैं।
प्रश्न 3.
“पूर्ण प्रतियोगिता में एक फ़र्म कीमत स्वीकार करने वाली क्यों होती है ?” स्पष्ट करें।
उत्तर-
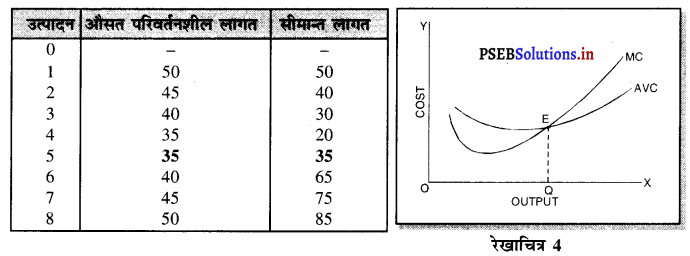
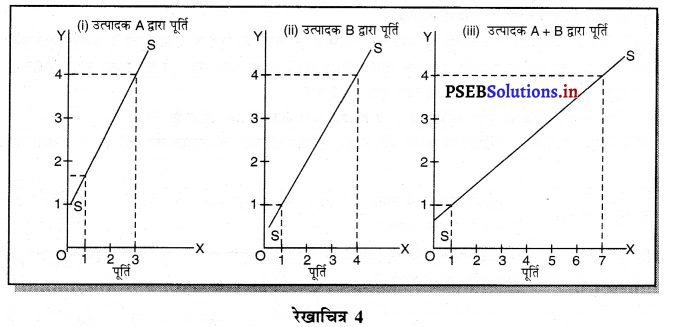
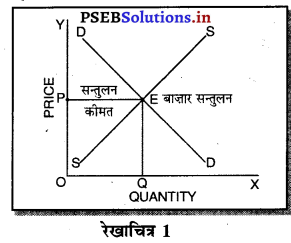
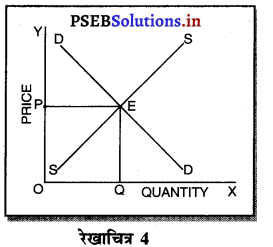
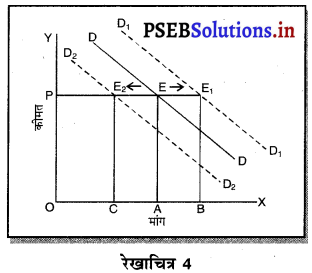
पूर्ण प्रतियोगिता में कीमत सभी उद्योगों में मांग तथा पूर्ति द्वारा निर्धारण होती है। इस कीमत को प्रत्येक फ़र्म स्वीकार करती है। इसलिए फ़र्म की औसत आय तथा सीमान्त आय सीधी रेखा होती है, जैसेकि रेखाचित्र 4 में दिखाया है। पूर्ण प्रतियोगिता में फ़र्म कीमत स्वीकार करने वाली क्यों होती है। इसके मुख्य कारण निम्नलिखित अनुसार हैं-
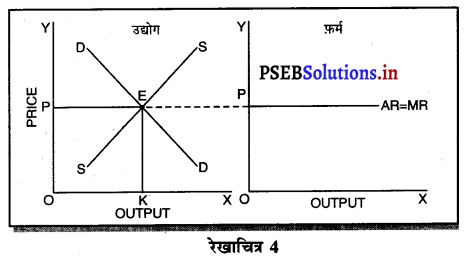
1. फ़र्मों की बड़ी संख्या (Large Number of Firms)-पूर्ण प्रतियोगिता में फ़र्मों की संख्या बहुत अधिक होती है। प्रत्येक फ़र्म का आकार इतना छोटा होता है कि बाजार में पूर्ति को प्रभावित नहीं कर सकती। इसलिए कीमत को प्रभावित नहीं कर सकती।
2. एक समान वस्तुएं (Homogeneous Product)-पूर्ण प्रतियोगिता में सभी फ़मैं एक समान तथा समरूप वस्तुओं का उत्पादन करती हैं। कोई फ़र्म यदि बाजार कीमत से अधिक कीमत निर्धारण करती है, तो उस फ़र्म की वस्तु बिकती नहीं। इसलिए बाज़ार में प्रचलित कीमत को ही स्वीकार करती है।
3. कम कीमत के कारण हानि (Loss due to less Price)- यदि फ़र्म बाजार कीमत से कम कीमत निश्चित करती है तो उसको हानि होगी, क्योंकि पूर्ण प्रतियोगिता में दीर्घकाल में सभी फ़र्मों को साधारण लाभ प्राप्त होता है। कीमत कम की जाती है तो हानि होगी।
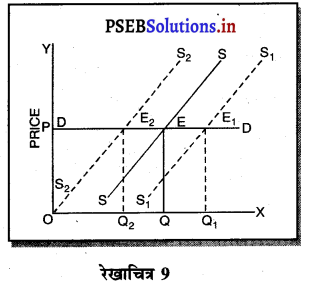
4. प्रवेश तथा छोड़ने की स्वतन्त्रता (Freedom of Entry and Exit)-पूर्ण प्रतियोगिता में यदि कुछ फ़र्मों को लाभ होता है तो नई फ़र्मे प्रवेश कर जाती है, जिससे कीमत कम हो जाती है। यदि कुछ फ़र्मों को हानि होती है तो वह फ़र्फे उद्योग को छोड़ जाती है, जिससे वस्तुओं की पूर्ति कम हो जाती है तथा कीमत बढ़ जाती है। इसलिए प्रत्येक फ़र्म उस कीमत को अपनाती है जो बाज़ार में उद्योग द्वारा निश्चित होती है।
![]()
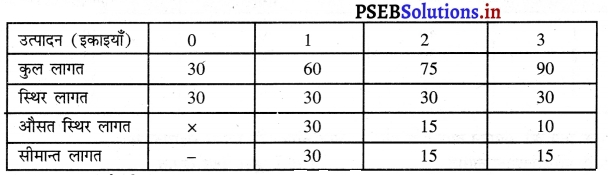
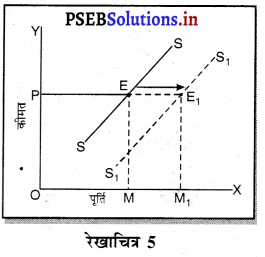
प्रश्न 4.
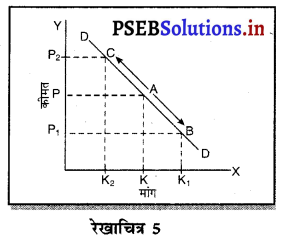
साधारण रूप में फ़र्म का अधिकतम MR = MC द्वारा क्यों होता है ? स्पष्ट करो।
उत्तर-
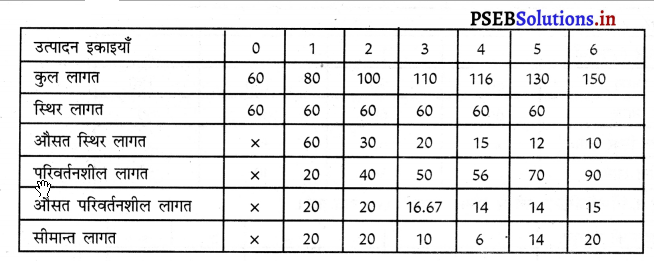
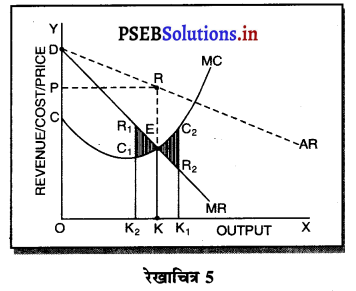
साधारण रूप में प्रत्येक फ़र्म को अधिकतम लाभ उस स्थिति में प्राप्त होता है, जहां फ़र्म की सीमान्त आय (MR) = सीमान्त लागत (MC) होती है। इसको रेखाचित्र को 5 द्वारा स्पष्ट किया जा सकता है। रेखाचित्र 5 में सन्तुलन MR = MC द्वारा सन्तुलन E बिन्दु पर होता है तथा फ़र्म OK उत्पादन करती है। कीमत OP निश्चित होती है।
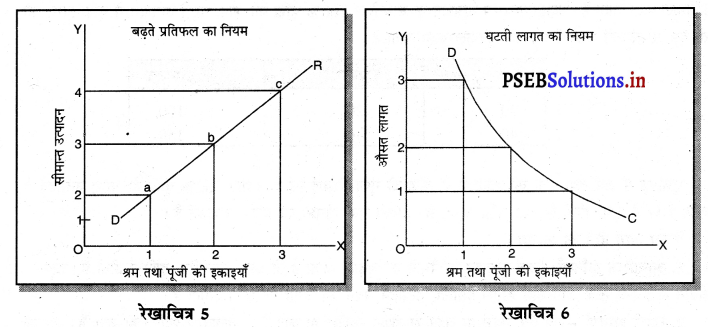
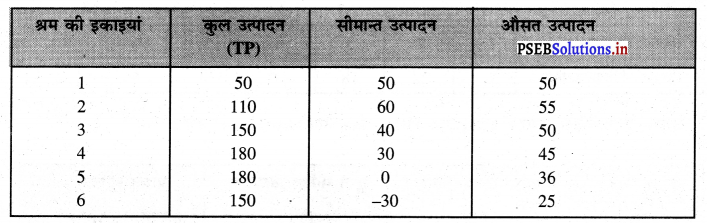
फ़र्म का लाभ DEC है जोकि अधिकतम है। यदि फ़र्म OK, वस्तुओं का उत्पादन करती है। लाभ DR1C1C प्राप्त होगा तथा DR1C1 लाभ DEC से कम है। यदि फ़र्म OK2 वस्तुओं का उत्पादन करती है तो OK उत्पादन तक लाभ DEC है तथा KK2 उत्पादन से EC2R2 हानि होगी, क्योंकि K2C2 सीमान्त लागत अधिक है तथा K2R2 सीमान्त आय कम है। DEC में से EC2R2 घटा दिया जाएं तो लाभ DEC MR से कम प्राप्त होगा। स्पष्ट है कि फ़र्म को अधिकतम लाभ उस स्थिति में होता है, जहां सीमान्त आय (MR) समान सीमान्त लागत (MC) होती है।
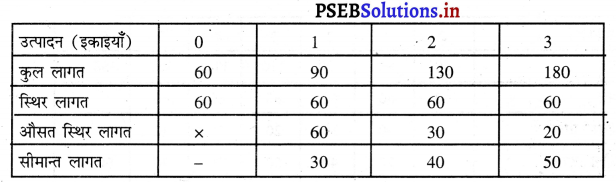
प्रश्न 5.
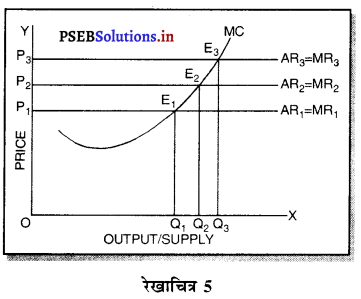
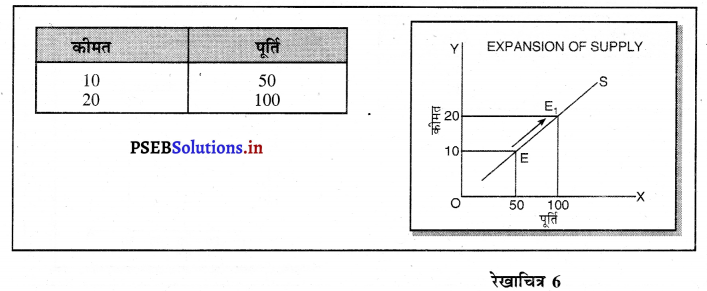
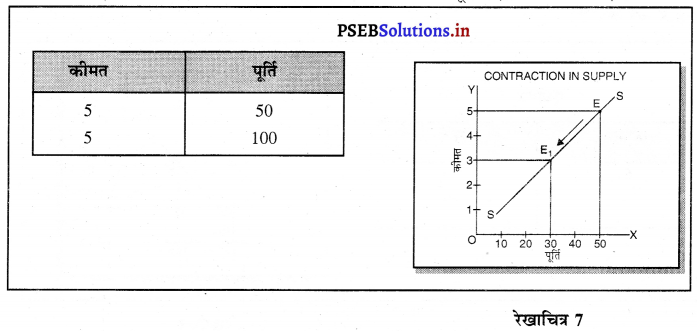
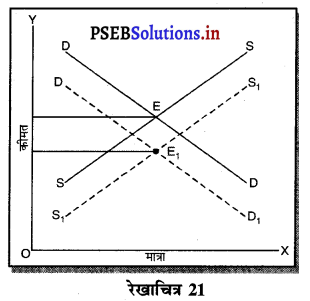
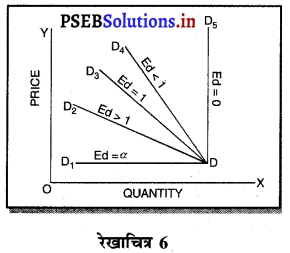
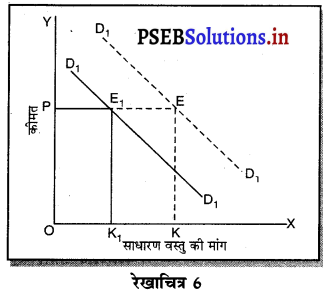
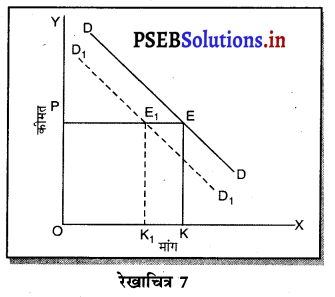
स्पष्ट करो कि पूर्ण प्रतियोगिता तथा दीर्घकाल में जब फ़र्मों के प्रवेश तथा छोड़ने की स्वतन्त्रता होती है तो फ़र्म को असाधारण लाभ शून्य कैसे होता है।
उत्तर-
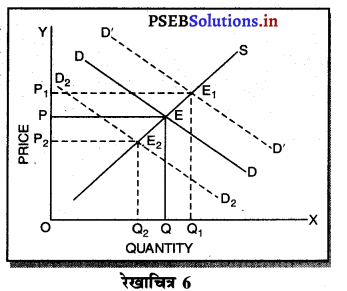
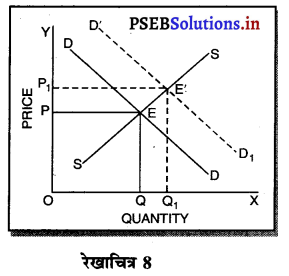
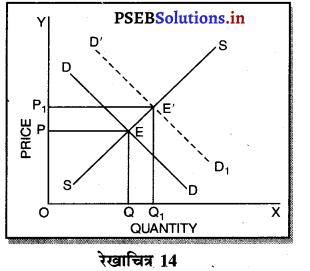
पूर्ण प्रतियोगिता तथा दीर्घ समय में जब फ़र्मों फ़र्म का सन्तुलन के निवेश तथा निकास की स्वतन्त्रता होती है तो उस स्थिति में केवल असाधारण लाभ अथवा साधारण प्राप्त | होता है। जैसे कि रेखाचित्र 6 द्वारा दिखाया गया है। रेखाचित्र 6 में जब कीमत उद्योग में कीमत OP, OP1 अथवा OP2 निश्चित होती है तो फ़र्म के सन्तुलन पर क्या प्रभाव पड़ता है। इसको स्पष्ट करते हैं।
(1) यदि कीमत OP निर्धारण होती है तो फ़र्म की मांग वक्र AR = MR सीधी रेखा बनती है। सन्तुलन MR = MC द्वारा E बिन्दु पर स्थापित होती है। OK उत्पादन किया जाता है। फ़र्म की AR = AC है। इसलिए साधारण लाभ होगा अथवा असाधारण लाभ शून्य होगा।
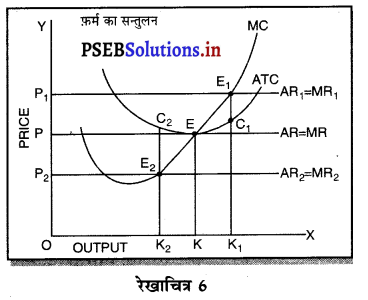
(2) यदि कीमत OP1 निर्धारण होती है तो दीर्घकाल में नई फ़र्में प्रवेश कर सकती हैं। इस स्थिति में सन्तुलन MR = MC द्वारा E, पर स्थापित होता है। फ़र्म OK1 उत्पादन करती है। फ़र्म की औसत आय K1E1 अधिक है, औसत लागत K1C1 कम है। इसलिए साधारण लाभ E1C1 होता है। परन्तु नई फ़र्मे प्रवेश करेगी। वस्तुओं की पूर्ति बढ़ जाएगी तथा कीमत OP1 से बढ़कर OP हो जाएगी। जहां कि असाधारण लाभ शून्य हो जाएगा।
(3) यदि कीमत OP2 निर्धारण होती है तो फ़र्म को हानि होगी। सन्तुलन MR = MC द्वारा E2 पर स्थापित होता है। K2C2 औसत लागत अधिक है। K2E2 औसत आय कम है। C2E2 हानि होगी। जिन फ़र्मों को हानि होती है। वह दीर्घकाल में उद्योग को छोड़ जाती है। वस्तुओं की पूर्ति कम हो जाएंगी। कीमत OP2 से बढ़कर OP हो जाती है। इस स्थिति में फ़र्म को साधारण लाभ होगा अथवा असाधारण लाभ शून्य हो जाता है।
B. एकाधिकार (Monopoly)
प्रश्न 6.
एकाधिकारी फ़र्म की बाज़ार मांग वक्र किस प्रकार की होती है ?
अथवा
एकाधिकार से क्या अभिप्राय है ? एक एकाधिकारी की मांग वक्र की प्रकृति का संक्षेप वर्णन करो।
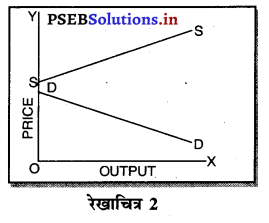
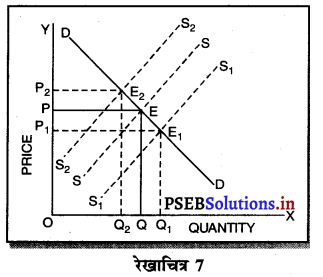
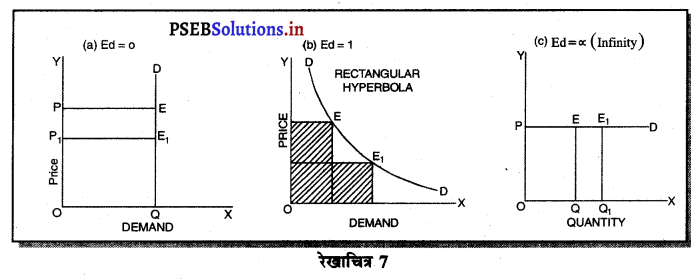
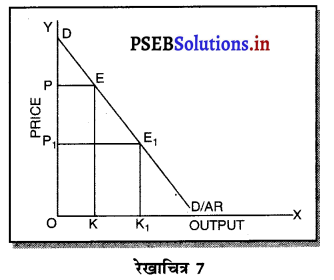
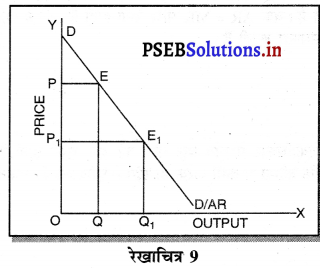
उत्तर-
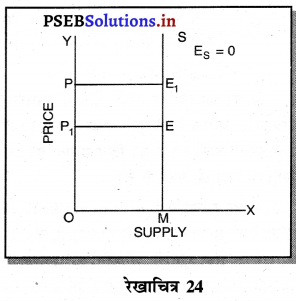
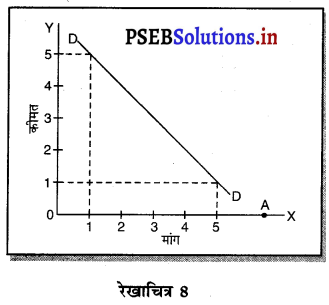
एकाधिकार (Monopoly) एक ऐसी स्थिति है, जिसमें वस्तु अथवा सेवा का एक उत्पादक होता है तथा जिस वस्तु का उत्पादन किया जाता है, उस वस्तु का नजदीकी स्थानापन्न नहीं होता। एकाधिकारी वस्तु की कीमत स्वयं निश्चित करता है। एकाधिकार में वस्तु की मांग (Demand Curve) ऋणात्मक ढाल वाली होती है। जैसे कि रेखाचित्र 7 में DD/ AR दिखाई गई है। जब कीमत OP है तो वस्तु की OK मात्रा की बिक्री की जाती है। यदि एकाधिकारी OK, वस्तु बेचनी चाहता है तो कीमत घटाकर OP, करनी पड़ती है। इससे मांग वक्र ऋणात्मक ढाल वाली बनती है। इसमें कीमत को घटाए बगैर वस्तु की अधिक मात्रा नहीं बेची जा सकती। इस कारण कीमत एक रुकावट होती है।
प्रश्न 7.
एकाधिकार बाज़ार संरचना का निर्माण कैसे होता है ?
उत्तर-
एकाधिकार बाजार संरचना का निर्माण निम्नलिखित ढंगों से होता है-
- सरकार द्वारा आज्ञा-सरकार किसी वस्तु के उत्पादन के लिए किसी फ़र्म को लाइसेस अथवा आज्ञा प्रदान करती है तो एकाधिकार का निर्माण होता है, जैसेकि डाकखाने, रेलवे, हवाई सेवा इत्यादि की एकाधिकारी।
- प्राकृतिक एकाधिकारी-एकाधिकार का निर्माण प्राकृति की ओर से भी हो सकता है। एक मनुष्य को ऐसा चश्मा मिल जाता है, जिसके पाने से हर बीमारी दूर हो जाती है तो इसको प्राकृतिक एकाधिकारी कहा जाता है।
- व्यापारिक गुण-बाज़ार में बहुत सी फ़में मिल जाती हैं तथा व्यापारिक गुट (cartels) बना लेती है। यह फ़र्मे वस्तु की कीमत तथा उत्पादन सम्बन्धी समझौता कर लेती हैं। जिससे एकाधिकारी का निर्माण होता है। कई बार सरकार ट्रस्ट विपक्षीय कानून (Anti-trust Legislation) भी बनाती है।
- पेटेंट अधिकार-कुछ फ़र्मे वस्तु का पेटेंट अधिकार प्राप्त कर लेती हैं। इसमें अन्य फ़र्मे उस वस्तु का निर्माण उसी रंग, रूप, आकार, स्वाद अथवा नाम इत्यादि से नहीं कर सकती।
प्रश्न 8.
पूर्ण प्रतियोगिता तथा एकाधिकार में मांग वक्र में अन्तर स्पष्ट करो।
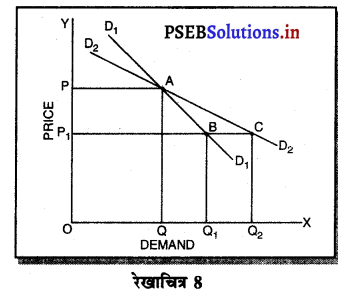
उत्तर-
1. पूर्ण प्रतियोगिता में मांग वक्र-पूर्ण प्रतियोगिता में मांग वक्र पूर्ण लोचशील तथा OX के समान्तर होती है। इससे अभिप्राय है कि OP कीमत पर एक फ़र्म OQ, OQ1 अथवा इससे अधिक जितनी चाहें वस्तुएं बेच सकती हैं। कीमत निर्धारण तो उद्योग द्वारा होती है, जिसको प्रत्येक फ़र्म अपना लेती है।
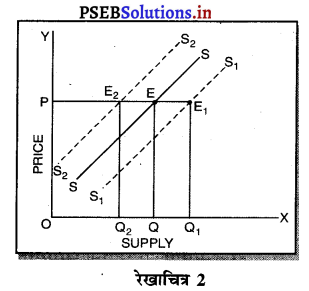
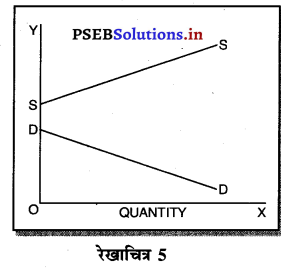
2. एकाधिकार में मांग वक्र-एकाधिकार में मांग वक्र बाईं से दाईं ओर नीचे की ओर झुकी रेखा होती है, जब मांग वक्र अथवा AR घंटती है तो MR तीव्रता से घटती है। इससे अभिप्राय है कि यदि कीमत OP से घटाकर OP1 की जाती है तो मांग OQ से बढ़कर OQ1 हो जाती है। इसलिए मांग में वृद्धि कीमत को घटाकर की जा सकती है।
प्रश्न 9.
एकाधिकार के मुख्य गुण तथा दोष बताओ।
उत्तर-
एकाधिकार के मुख्य गुण (Merits) निम्नलिखित हैं-
- कार्यकुशलता में वृद्धि-जब एकाधिकार स्थापित हो जाता है तो उत्पादन बड़े पैमाने पर होता है इससे पैमाने की बचतें प्राप्त होती हैं। तकनीक का विकास किया जाता है। श्रम के विभाजन के लाभ प्राप्त होते हैं। इसीलिए कार्यकुशलता में वृद्धि होती है।
- अनुसन्धान तथा विकास का ऊंचा स्तर-एकाधिकार फ़र्म के पास साधन बहुत अधिक होते हैं। इसलिए यह फ़र्म वस्तु के विकास के लिए नए अनुसन्धानों पर व्यय कर सकती है। इससे उत्पादन लागत में कमी होती है।
एकाधिकार के दोष (Demerits of Monopoly)-
1. ऊंची कीमत-एकाधिकार बाज़ार में विक्रेता एक फ़र्म होती है जोकि अधिकतम लाभ के उद्देश्य से उत्पादन करती है। इसलिए कीमत निर्धारण इस प्रकार की जाती है, जिसमें उस फ़र्म द्वारा निश्चित की कीमत फ़र्म की सीमान्त लागत से अधिक (P> MC) होती है।
2. कम उत्पादन-एकाधिकार द्वारा जो उत्पादन किया जाता है वह पूर्ण प्रतियोगिता की तुलना में कम होता है, चाहे सन्तुलन MR = MC द्वारा स्थापित किया जाता है, परन्तु पूर्ण प्रतियोगिता में AR = MR सीधी रेखा होती है, परन्तु एकाधिकार में AR घटती है तो MR तीव्रता से घटती है, इसलिए सन्तुलन की स्थिति में एकाधिकार का उत्पादन कम होता है।
3. केन्द्रीयकरण को उत्साह-एकाधिकार के पास आर्थिक शक्ति बढ़ जाती है। एक बार एकाधिकार प्राप्त करने के पश्चात् यह फ़र्म किसी अन्य फ़र्म को बाज़ार में आने नहीं देती। इससे शोषण में वृद्धि होती है तथा बाज़ार पर एकाधिकारी का नियन्त्रण हो जाता है।
![]()
प्रश्न 10.
पूर्ण प्रतियोगिता तथा एकाधिकार बाज़ार में अन्तर स्पष्ट करो।
उत्तर-
पूर्ण प्रतियोगिता तथा एकाधिकार बाज़ार में मुख्य अन्तर इस प्रकार है –
1. खरीददारों तथा बेचने वालों की संख्या-पूर्ण प्रतियोगिता में खरीददारों तथा बेचने वालों की संख्या बहुत अधिक होती है। एकाधिकार में बेचने के लिए एक फ़र्म तथा खरीददार अधिक होते हैं।
2. वस्तुओं की प्रकृति-पूर्ण प्रतियोगिता में प्रत्येक फ़र्म समरूप वस्तु (Homogenous Products) का उत्पादन करती है। परन्तु एकाधिकार में ऐसी वस्तु का उत्पादन किया जाता है जिसका कोई नज़दीकी स्थानापन्न नहीं होता। (No close substitute)
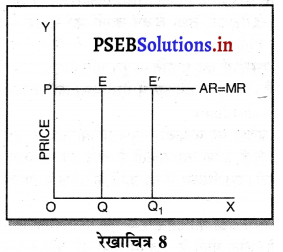
3. प्रवेश पर पाबन्दी-पूर्ण प्रतियोगिता में फ़मैं उद्योग मांग वक्र में जब चाहें प्रवेश कर सकती है तथा उन पर कोई पाबन्दी नहीं होती। एकाधिकार में नई फ़र्मों के प्रवेश पर पाबन्दी होती है।
4.मांग वक्र का आकार-पूर्ण प्रतियोगिता में मांग वक्र पूर्ण लोचशील होती है, जिसमें वस्तु की कीमत एक समान रहती है। वक्र AR = MR सीधी रेखा होती है, जोकि Ox के समान्तर बनती है।
एकाधिकार में मांग वक्र नीचे की ओर झुकी होती है, क्योंकि कीमत में कमी करके ही मांग में वृद्धि की जा सकती है।
 5. कीमत विभेद-पूर्ण प्रतियोगिता में कीमत विभेद सम्भव नहीं होता क्योंकि प्रत्येक फ़र्म एक समान कीमत निश्चित करती है। एकाधिकार में कीमत विभेद सम्भव होता है।
5. कीमत विभेद-पूर्ण प्रतियोगिता में कीमत विभेद सम्भव नहीं होता क्योंकि प्रत्येक फ़र्म एक समान कीमत निश्चित करती है। एकाधिकार में कीमत विभेद सम्भव होता है।
प्रश्न 11.
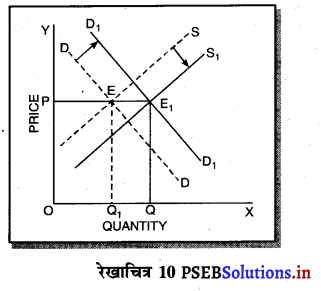
एकाधिकार में दीर्घकाल में असाधारण लाभ प्राप्त किया जाता है। स्पष्ट करो।
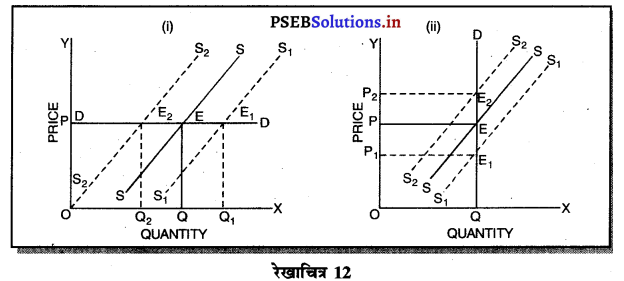
उत्तर-
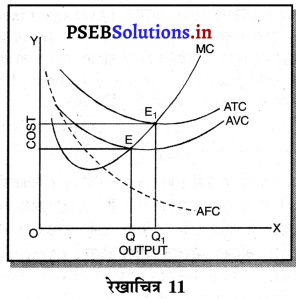
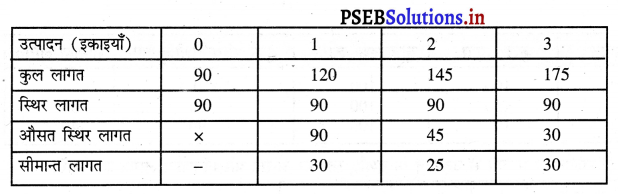
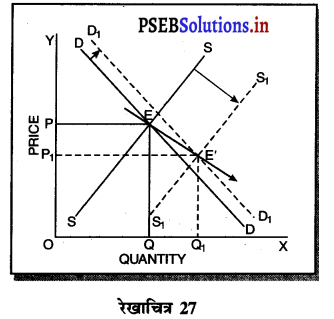
दीर्घकाल में सन्तुलन में एकाधिकारी फ़र्म असाधारण लाभ (Abnormal Profit) प्राप्त करती है। इसको रेखाचित्र 12 द्वारा स्पष्ट करते हैं। रेखाचित्र 12 में आय रेखाएं AR तथा MR है जोकि घटती रेखाएं हैं। दीर्घकाल की लागत रेखाएं (ATC तथा LMC है। सन्तुलन MR = LMC द्वारा E बिन्दु पर होता है। OQ LMC उत्पादन किया जाता है। QR = OP कीमत निर्धारण हो जाती है। औसत लागत QT = OC दिखाई गई है। इस स्थिति में PRTC असाधारण लाभ प्राप्त होता है। एकाधिकार में दीर्घकाल में साधारण से अधिक लाभ प्राप्त होते हैं।
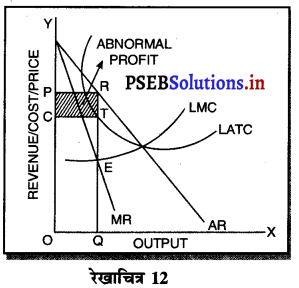
(C) एकाधिकारी प्रतियोगिता (Monopolistic Competition)
प्रश्न 12.
एकाधिकारी प्रतियोगिता की विशेषताओं का संक्षेप वर्णन करो।
उत्तर-
एकाधिकार प्रतियोगिता की मुख्य विशेषताएं निम्नलिखित हैं-
1. खरीददारों तथा बेचने वालों की बड़ी संख्या-आधुनिक युग में बाज़ार को एकाधिकारी प्रतियोगिता कहा जाता है। इसमें बेचने वालों की संख्या 20 तक होती है तथा खरीददारों की संख्या बहुत अधिक है।
2. वस्तु भिन्नता- इस बाज़ार की सबसे प्रमुख विशेषता वस्तु भिन्नता (Product differentiation) होती है। वस्तु भिन्नता का अर्थ है कि प्रत्येक उत्पादक वस्तु के रंग, रूप, आकार, स्वाद इत्यादि में परिवर्तन करके अपनी वस्तु को भिन्न बनाने का प्रयत्न करता है।
3. प्रवेश तथा छोड़ने की स्वतन्त्रता-इस बाज़ार में फ़र्मों को किसी उद्योग में प्रवेश करने अथवा किसी उद्योग को छोड़ने की आज्ञा होती है। जब किसी उद्योग में लाभ प्राप्त होता है तो नई फ़ प्रवेश कर सकती हैं। हानि की स्थिति में फ़र्म उद्योग को छोड़ सकती हैं।
4. बिक्री लागतें-इस बाज़ार में प्रचार पर व्यय किया जाता है। जो व्यय प्रचार पर होता है, उसको बिक्री लागत कहा जाता है। इससे वस्तु की बिक्री बढ़ जाती है।
प्रश्न 13.
एकाधिकार अथवा एकाधिकार प्रतियोगिता में कीमत सीमान्त लागत से अधिक कैसे होती है रेखा चित्र स्पष्ट करो ?
उत्तर-
एकाधिकार अथवा एकाधिकार प्रतियोगिता में कीमत सीमान्त लागत से अधिक होती है। इन दोनों बाजारों में औसत आय (AR) तथा सीमान्त आय (MR) नीचे को घटती रेखाएं होती हैं। जब औसत आय घटती है तो सीमान्त आय तीव्रता से घटती है। प्रत्येक फ़र्म का मुख्य उद्देश्य अधिकतम लाभ प्राप्त करना होता है, जोकि सीमान्त आय MR तथा सीमान्त लागत MC के समान होने से प्राप्त होता है। (MR = MC) क्योंकि AR > MR होती है, इसलिए Por AR > MC होगी। जैसेकि रेखाचित्र 13 में स्पष्ट किया गया है कि औसत आय तथा सीमान्त आय घटती रेखाएं हैं। सन्तुलन MR = MC द्वारा E बिन्दु पर स्थापित होता है। OQ उत्पादन किया जाता है। फ़र्म की औसत आय QR है अथवा कीमत OP निर्धारण की जाती है। हम देखते हैं कि OP or QR > QE अर्थात् कीमत, सीमान्त लागत से अधिक है ।
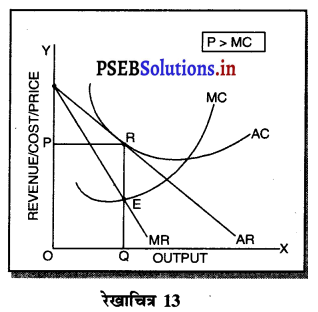
प्रश्न 14.
स्पष्ट करो कि एकाधिकार प्रतियोगिता में मांग वक्र अधिक लोचशील होती है।
अथवा
स्पष्ट करो कि एकाधिकार प्रतियोगिता में मांग वक्र पूर्ण लोचशील से कम तथा पूर्ण प्रतियोगिता में पूर्ण लोचशील होती है।
उत्तर-
पूर्ण प्रतियोगिता में मांग पूर्ण लोचशील होती है। क्योंकि इस बाज़ार में कीमत उद्योग में मांग तथा पूर्ति द्वारा निर्धारण होती है, जिसको प्रत्येक फ़र्म अपना लेती है। इस कीमत पर फ़र्म जितनी चाहें वस्तु की मात्रा बेच सकती है। इसलिए मांग वक्र ox के समान्तर होती है।
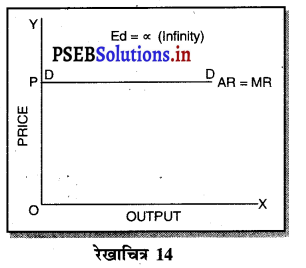
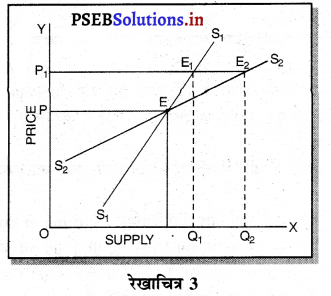
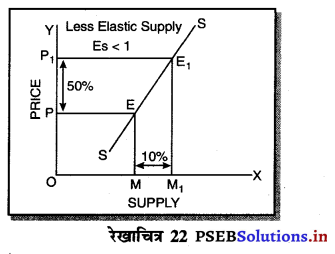
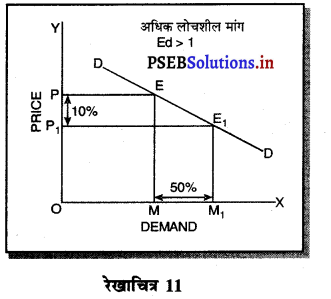
एकाधिकार प्रतियोगिता में मांग अधिक लोचशील होती है, क्योंकि इस बाजार में वस्तुएं एक-दूसरे की स्थानापन्न होती हैं। वस्तु की कीमत में थोड़ी-सी कमी PP1 से मांग में बहुत अधिक वृद्धि QQ1 होती है। इसीलिए मांग वक्र अधिक लोचशील होती है। परन्तु यह पूर्ण लोचशील नहीं, क्योंकि PD पूर्ण प्रतियोगिता में दिखाई है। बल्कि पूर्ण लोचशील से कम है।
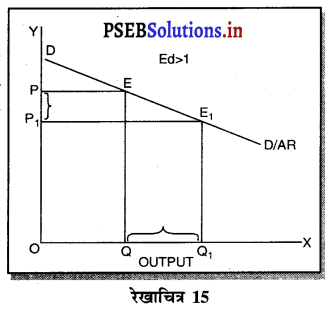
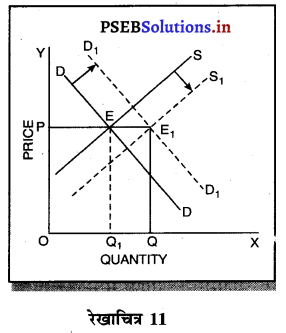
प्रश्न 15.
स्पष्ट करो कि दीर्घकाल में स्वतन्त्र प्रवेश तथा छोड़ने से एकाधिकारी प्रतियोगिता में असाधारण लाभ शून्य प्राप्त होता है ?
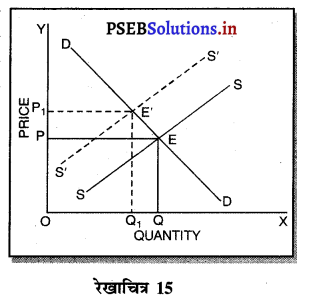
उत्तर-
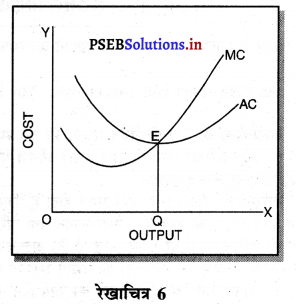
एकाधिकारी प्रतियोगिता में दीर्घ काल में सभी फ़र्मों के असाधारण लाभ शून्य प्राप्त होते हैं, क्योंकि-
1. पूर्ति में वृद्धि–यदि एकाधिकारी प्रतियोगिता में फ़र्मों को असाधारण लाभ प्राप्त होता है तो नई फ़र्मों के प्रवेश से वस्तुओं की पूर्ति बढ़ जाती है। इससे कीमत कम हो जाती है तथा असाधारण लाभ शून्य हो जाता है।
2. लागत में वृद्धि-जब नई फ़र्मे प्रवेश करती हैं तो इससे उत्पादन के साधनों की लागत बढ़ जाती है। पुरानी फ़र्मों की लागत बढ़ने के कारण उनके असाधारण लाभ शून्य हो जाते हैं।
3. कीमत में कमी-जब एकाधिकारी प्रतियोगिता में नई फ़में प्रवेश करती हैं तो वस्तु बेचने की कीमत कम निर्धारण करती हैं ताकि जो वस्तु की मांग में वृद्धि हों। पुरानी फ़र्मों को भी वस्तु की कीमत घटानी पड़ती है तथा उनको असाधारण लाभ शून्य प्राप्त होता है।
4. हानि-यदि कुछ फ़र्मों को हानि होती है तो वह फ़र्मों उद्योग को छोड़कर चली जाती हैं। इससे वस्तुओं की पूर्ति कम हो जाती है। कीमत बढ़ जाती है तथा फ़र्मों को साधारण लाभ ही प्राप्त होता है। रेखाचित्र 16 में फ़र्म के दीर्घकाल के सन्तुलन को स्पष्ट किया गया है। सन्तुलन MR = LMC द्वारा स्थापित होता है। फ़र्म OQ उत्पादन करती है। OR औसत आय के समान है। इसलिए प्रत्येक फ़र्म को साधारण लाभ प्राप्त होते हैं।
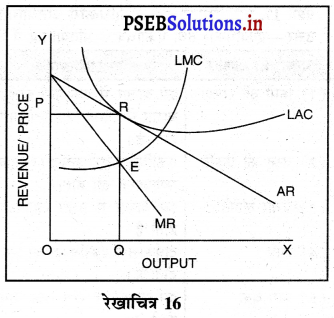
प्रश्न 16.
पूर्ण प्रतियोगिता तथा एकाधिकारी प्रतियोगिता में अन्तर स्पष्ट करो।
उत्तर–
पूर्ण प्रतियोगिता तथा एकाधिकारी प्रतियोगिता में अन्तर इस प्रकार है –
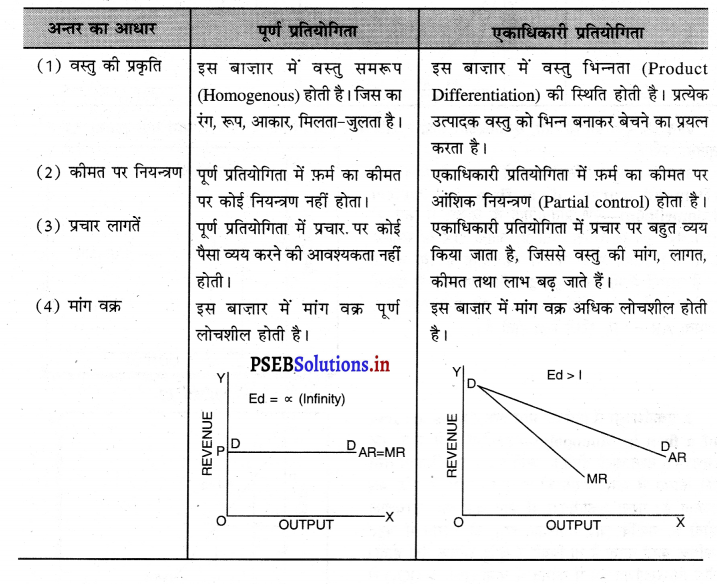
![]()
प्रश्न 17.
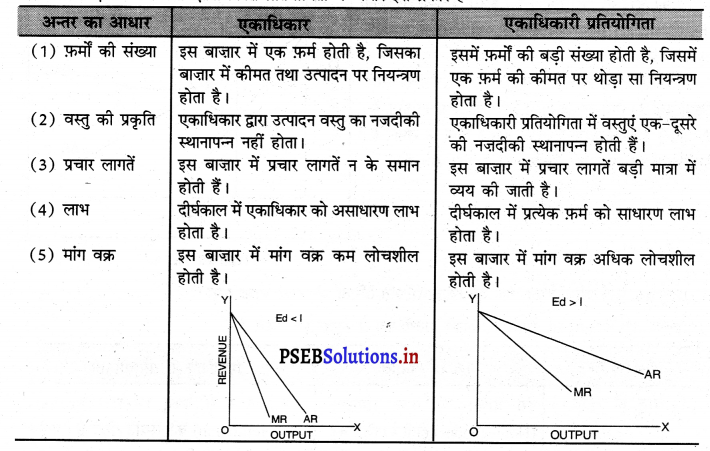
एकाधिकार तथा एकाधिकारी प्रतियोगिता में अन्तर स्पष्ट करो।
उत्तर-
एकाधिकार तथा एकाधिकारी प्रतियोगिता में अन्तर इस प्रकार है –
प्रश्न 18.
पूर्ण प्रतियोगिता, एकाधिकार तथा एकाधिकार प्रतियोगिता में फ़र्म के मांग वक्र अथवा AR की तुलना करो।
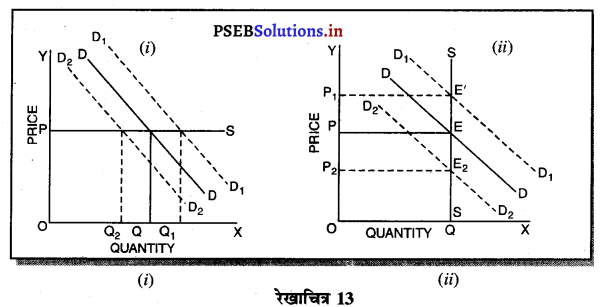
उत्तर-
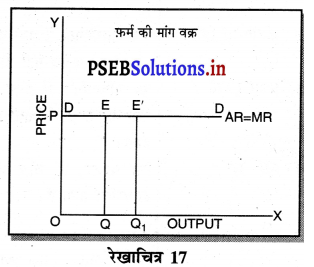
1. पूर्ण प्रतियोगिता में फ़र्म की मांग वक्र फ़र्म की मांग वक्र (Demand curve of a firm in Perfect Competition)-पूर्ण प्रतियोगिता के बाज़ार में फ़र्म की मांग वक्र पूर्ण लोचशील होती है, जोकि OX के समान्तर होती है। इसका मुख्य कारण यह होता है कि कीमत AR=MR सभी उद्योगों में मांग तथा पूर्ति द्वारा निर्धारण होती है, इसको प्रत्येक फ़र्म अपना लेती है। फ़र्म की मांग वक्र DD अथवा AR = MR सीधी रेखा होती है।
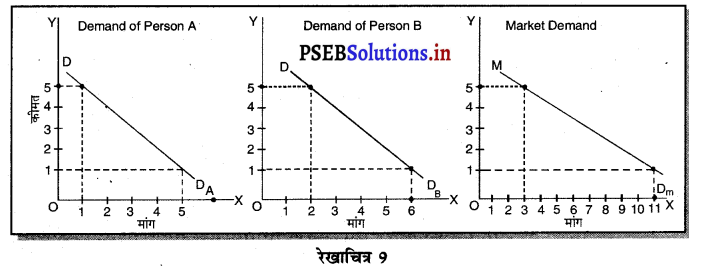
2. एकाधिकार में फ़र्म की मांग वक्र (Demand curve of a firm in Monopoly)-एकाधिकार में मांग वक्र ऋणात्मक ढाल वाली होती है, क्योंकि एक एकाधिकारी वस्तु की कीमत में कमी करके ही वस्तु की मांग में वृद्धि कर सकता है। परन्तु एकाधिकार में मांग वक्र कम लोचशील होती है, क्योंकि यदि उत्पादक वस्तु की कीमत में बहुत अधिक कमी करता है तो बिक्री में वृद्धि अधिक नहीं होती। जैसे कि रेखाचित्र 18 में कीमत में कमी (PP1 > QQ1) से उत्पादन में वृद्धि कम अनुपात पर होती है।
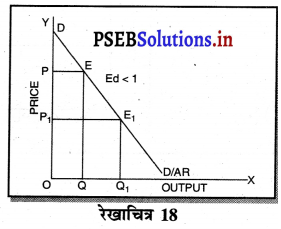
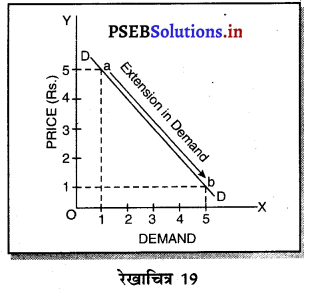
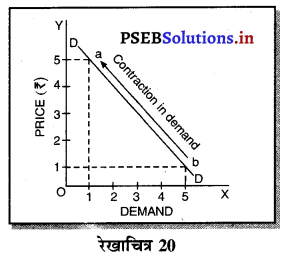
3. एकाधिकारी प्रतियोगिता में फ़र्म की मांग वक्र (Demand Curve of a firm in Monopolistic Competition)-एकाधिकारी प्रतियोगिता में मांग वक्र ऋणात्मक ढलान वाली अधिक लोचशील है। जैसे कि रेखाचित्र 19 में DD अथवा AR नीचे की ओर जाती वक्र दिखाई गई है अर्थात् यदि कीमत PP1 थोड़ी-सी कम हो जाती है तो मांग में वृद्धि QQ1 अधिक होती है। इसका मुख्य कारण प्रत्येक उत्पादक की वस्तु दूसरे उत्पादकों का नज़दीकी स्थानापन्न होती है। इसलिए एक उत्पादक द्वारा थोड़ी सी कीमत घटाने से मांग बहुत बढ़ जाती है।
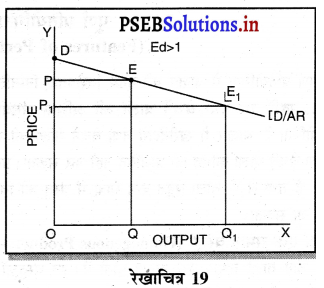
प्रश्न 19.
पूर्ण प्रतियोगिता में फ़र्म का कीमत पर कोई नियन्त्रण नहीं होता, जबकि एकाधिकारी प्रतियोगिता में फ़र्म का कीमत पर आंशिक नियन्त्रण होता है। स्पष्ट करो।
उत्तर-
पूर्ण प्रतियोगिता में कीमत मांग तथा पूर्ति द्वारा उद्योग में निर्धारण होती है, जो कीमत उद्योग में निर्धारण हो जाती है, वह कीमत प्रत्येक फ़र्म को अपनानी पड़ती है, क्योंकि इस बाज़ार में प्रत्येक फर्म समरूप वस्तुओं का उत्पादन करती है। इसलिए फ़र्म वस्तु की कीमत में परिवर्तन नहीं कर सकती तथा उसका कीमत पर कोई नियन्त्रण नहीं होता।
एकाधिकारी प्रतियोगिता में वस्तु भिन्नता पाई जाती है। इसलिए प्रत्येक फ़र्म अपनी वस्तु के रंग, रूप, आकार, स्वाद, प्रचार इत्यादि द्वारा भिन्न बनाकर बेचने का प्रयत्न करती है। इसलिए एकाधिकारी प्रतियोगिता में फ़र्म अपनी वस्तु की विभिन्न कीमत रख सकती है। परन्तु इस बाज़ार में वस्तुएं एक-दूसरे की नज़दीकी स्थानापन्न होती हैं। इसलिए कीमत में अधिक परिवर्तन नहीं किया जा सकता। बल्कि थोड़ी-सा परिवर्तन सम्भव होता है। इस कारण फ़र्म का कीमत पर आंशिक नियन्त्रण होता है।
‘
(D) अल्प अधिकार (Oligopoly)
प्रश्न 20.
अल्प-अधिकार की चार विशेषताएं बताएं।
उत्तर-
अल्प-अधिकार आज कल के युग में एक व्यावहारिक बाज़ार है। इसमें कम फर्मे (A few Firms) होती हैं। यह फर्मे एक समान वस्तुओं का उत्पादन करती हैं जैसे कि सीमेंट, आटा आदि अथवा एक दूसरे के नज़दीक की वस्तुओं का उत्पादन करती हैं। इस बाज़ार में विक्रेताओं की संख्या 2 से 10 तक होती है जो विलियम फैलनर के अनुसार, “अल्प-अधिकार कम फर्मों में प्रतियोगिता का बाज़ार होता है।” (Oligopoly is a market with competition among the few. -Fellner)
अल्प-अधिकार की विशेषताएं (Features of Oligopoly) –
- आत्मनिर्भरता (Independence)-इस बाज़ार में फर्मे एक-दूसरे पर निर्भर होती हैं। एक फर्म का निर्णय दूसरी फर्मों को प्रभावित करता है। यदि एक फर्म कीमत कम कर देती है तो दूसरी फर्म को भी कीमत कम करनी पड़ती
- प्रचार (Advertisement)-इस बाज़ार में प्रचार पर बहुत व्यय किया जाता है। प्रचार करके प्रत्येक उत्पादक अधिक बिक्री करने का यत्न करता है।
- प्रतियोगिता (Competition)- इस बाज़ार में फर्मों में प्रतियोगिता पाई जाती है। यदि एक उत्पादक वस्तु के साथ कोई तोहफा देता है तो दूसरे उत्पादकों को भी ऐसा ही करना पड़ता है।
- मांग वक्र (Demand Curve)-इस बज़ार में मांग वक्र स्पष्ट नहीं होती। एक फर्म द्वारा कीमत कम अथवा अधिक करने से दूसरी फर्मे भी वैसा ही करती हैं। टेढ़ी मांग वक्र इसलिए माँग वक्र टेढ़ी रेखा होती है।
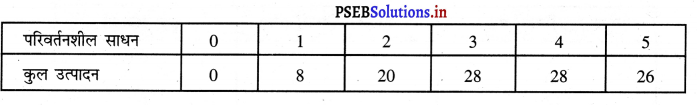
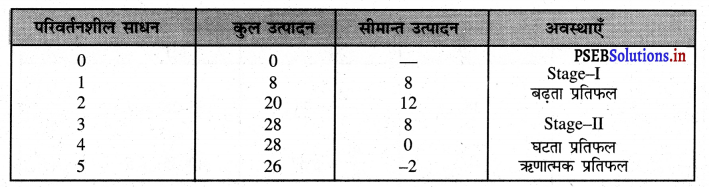
- एकसारता का अभाव (No Uniformity)प्रत्येक फर्म का आकार अलग होता है। एक फर्म बढ़ी तथा दूसरी फर्मे छोटे आकार की हो सकती हैं।
- समूह व्यवहार (Group Behaviour)-प्रत्येक फर्म का अपना विशेष बाज़ार होता है। नई फमें आ सकती हैं। परन्तु जो फर्मे लगी होती हैं वह आसानी से फर्मों को आने नहीं देतीं।
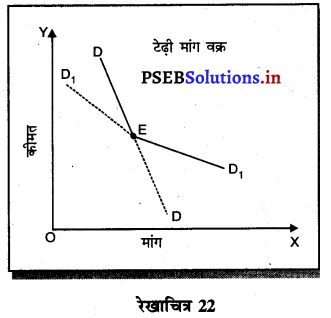
IV. दीर्घ उत्तरीय प्रश्न (Long Answer Type Questions)
प्रश्न 1.
पूर्ण प्रतियोगिता के बाज़ार से क्या अभिप्राय है ? पूर्ण प्रतियोगिता की विशेषताएं बताओ। (What is Perfectly Competitive Market ? Explain the main features of Perfect Competition.)
उत्तर-
पूर्ण प्रतियोगिता का अर्थ (Meaning of Perfect Competition)-पूर्ण प्रतियोगिता बाज़ार की वह अवस्था है जहां कि वस्तु के खरीददार तथा बेचने वाले बहुत अधिक होते हैं। एक समान वस्तुएं (Homogenous) का उत्पादन किया जाता है। खरीददारों तथा बेचने वालों में प्रतियोगिता इस ढंग से होती है कि सारे बाज़ार में एक वस्तु की एक समय सीमा निश्चित हो जाती है। वस्तु की कीमत उद्योग द्वारा मांग तथा पूर्ति की शक्तियों द्वारा निर्धारित हो जाती है, जिसको प्रत्येक फ़र्म स्वीकार करती है। प्रो० बिलास के अनुसार, “पूर्ण प्रतियोगिता एक ऐसी दशा है, जिसमें बहुत सी फ़में होती हैं। सभी फ़मैं एक समान वस्तुओं का उत्पादन करती है तथा फ़र्म कीमत स्वीकार करने वाली होती है।” (“Perfect competition is characterised by presence of many firms. All the firms sell identical products and a firm is a price taper.”-Bilas)
पूर्ण प्रतियोगिता की विशेषताएं (Features of Perfect Competition) –
पूर्ण प्रतियोगिता में बाज़ार की मुख्य विशेषताएं निम्नलिखित अनुसार है-
1. खरीददार तथा बेचने वालों की अधिक संख्या (Large number of Buyers and Sellers)-पूर्ण प्रतियोगिता के बाजार में खरीददारों तथा बेचने वालों की संख्या बहुत अधिक होती है अर्थात् कोई भी खरीदने वाला अथवा बेचने वाला कीमत को प्रभावित नहीं कर सकता। प्रत्येक खरीददार बाज़ार में बहुत कम मात्रा में वस्तु की खरीद करता है तथा बेचने वाला बहुत कम मात्रा में वस्तु की बिक्री करता है, जिससे वस्तु की कीमत पर कोई प्रभाव नहीं पाया जा सकता।
2. एक समान वस्तुएं (Homogenous Products)-पूर्ण प्रतियोगिता के बाज़ार में भिन्न-भिन्न उत्पादकों द्वारा बेची जाने वाली वस्तुएं एक समान होती हैं। इन वस्तुओं का रंग-रूप, आकार, स्वाद इत्यादि एक समान होता है। इसलिए खरीददार किसी भी उत्पादक द्वारा उत्पादन की वस्तु को खरीद सकते हैं। पूर्ण प्रतियोगिता के बाजार की एक विशेष विशेषता है।
3..फर्मों को उद्योग में प्रवेश तथा छोड़ने की स्वतन्त्रता (Freedom of entry and exist of firms)-पूर्ण प्रतियोगिता में फ़र्मे बाज़ार में जब चाहे प्रवेश कर सकती हैं तथा उन्हें बाजार को छोड़ने की पूर्ण स्वतन्त्रता होती है। बाज़ार में सरकार कोई हस्तक्षेप नहीं करती अर्थात् फ़में किसी भी समय वस्तु का उत्पादकता आरम्भ कर सकती हैं तथा किसी भी समय फ़र्मों को बन्द कर सकती है। नई फ़र्मों के खुलने तथा पुरानी फ़र्मों के बन्द होने पर कोई प्रतिबन्ध नहीं होता।
4. पूर्ण ज्ञान (Perfect Knowledge)-इस बाज़ार में वस्तुएं बेचने वालों को तथा वस्तुएं खरीदने वालों को पूर्ण ज्ञान होता है। उनको यह ज्ञात होता है कि बाज़ार के किस भाग में किस कीमत पर वस्तु के सौदे हो रहे हैं। न तो बेचने वाला बाजार की अज्ञानता के कारण वस्तु को कम कीमत पर बेचता है तथा न ही खरीदने वाला वस्तुओं को अधिक कीमत पर खरीदता है।
5. पूर्ण गतिशीलता (Perfect Mobility)-पूर्ण प्रतियोगिता के बाज़ार में उत्पादन के साधनों में पूर्ण गतिशीलता पाई जाती है, जिन कार्यों में उत्पादन के साधनों की मांग अधिक होती है तथा कीमत बढ़ा दी जाती है। उन कार्यों में उत्पादन के साधनों की पूर्ति बढ़ जाती है। इस प्रकार उत्पादन के साधन एक उद्योग से दूसरे उद्योग में जाने के लिए स्वतन्त्र होते हैं।
6. यातायात व्यय का न होना (No Transportation Cost)-पूर्ण प्रतियोगिता की स्थिति में यातायात की लागत नहीं होती। यदि फ़र्मों को यातायात का व्यय सहन करना पड़ता है तथा इस व्यय को कीमत में शामिल किया जाता है तो विभिन्न स्थानों पर वस्तु विभिन्न होने की सम्भावना होती है। परन्तु पूर्ण प्रतियोगिता में वस्तु की एक कीमत निश्चित होती है। इसलिए यातायात के साधनों की लागत को वस्तु की कीमत में शामिल नहीं किया जाता।
7. मांग वक्र (Demand curve)-पूर्ण प्रतियोगिता के बाज़ार में मांग वक्र पूर्ण लोचशील होती है, जोकि OX रेखा के समान्तर होती है। इस बाज़ार में फ़र्म कीमत स्वीकार करने वाली (Firm is a Price taker) होती है।
प्रश्न 2.
एकाधिकार से क्या अभिप्राय है ? एकाधिकार की मुख्य विशेषताओं का वर्णन करो।
(What is Monopoly ? Explain the characteristics of Monopoly.)
उत्तर-
एकाधिकार शब्द लैटिन भाषा के दो शब्दों से मिलकर बना है, मोनो (Mono) तथा पोली (Poly)। मोनो का अर्थ है एक तथा पोली का अर्थ है बेचने वाला अर्थात् एकाधिकार से अभिप्राय है कि मंडी की वह स्थिति जिसमें वस्तु को बेचने वाला एक हो तथा खरीदने वाले बहुत से हों। बाजार में जो वस्तु एकाधिकार द्वारा उत्पादन की जाती है, उस वस्तु का नज़दीकी स्थानापन्न नहीं होना चाहिए। इसलिए बाज़ार में कोई प्रतियोगिता नहीं होती।
(Monopoly is a market in which there is only one producer, producing a commodity which has no close substitute.) .
इससे स्पष्ट होता है कि एकाधिकार उस बाजार को कहा जाता है, जिसमें वस्तु को पैदा करने वाला एक व्यक्ति होता है, जिस वस्तु का उत्पादन किया जाता है, उसका नज़दीकी स्थानापन्न प्राप्त नहीं होता। कोई फ़र्म उद्योग में प्रवेश नहीं कर सकती।
एकाधिकार की विशेषताएं (Characteristics of Monopoly)-
1. एक उत्पादक (One Producer)-एकाधिकार बाज़ार में वस्तु का उत्पादन एक व्यक्ति होता है, जबकि वस्तु . के खरीददार बहुत से होते हैं। इसमें एक मनुष्य अकेला भी फ़र्म का संचालन कर सकता है अथवा साझीदार बनाकर एकाधिकारी स्थापित की जा सकती है, परन्तु वस्तु का उत्पादन एक फ़र्म के हाथ में ही होता है।
2. नज़दीकी स्थानापन्न नहीं होता (No Close Substitute)-एकाधिकारी बाज़ार की यह एक महत्त्वपूर्ण विशेषता है कि जो वस्तु एकाधिकारी द्वारा पैदा की जाती है। उस वस्तु का बाज़ार में कोई भी स्थानापन्न नहीं होता। इस कारण कोई नई फ़र्म बाज़ार में शामिल नहीं हो सकती, क्योंकि उस वस्तु की जगह पर प्रयोग की जाने वाली वस्तु का उत्पादन नहीं किया जा सकता।
3. प्रतियोगिता का अभाव (No Competition) एकाधिकारी बाज़ार में प्रतियोगिता का अभाव होता है। कोई नई फ़र्म वस्तु की पैदावार नहीं कर सकती। इसलिए एकाधिकारी फ़र्म वस्तु की मर्जी से कीमत निश्चित कर सकती है।
4. वस्तु की कीमत पर नियन्त्रण (Control over Price)-बाज़ार में एकाधिकारी अकेला ही उत्पादक होता है। इसलिए वस्तु की कीमत पर उसका पूरा नियन्त्रण होता है। जब चाहे वस्तु की कीमत में वृद्धि अथवा कमी कर सकता है। देश में विभिन्न वर्ग के लोगों से वस्तु की विभिन्न कीमत भी प्राप्त की जा सकती है।
5. एकाधिकार फ़र्म उद्योग भी होते हैं (Firm is also an Industry) बाज़ार में वस्तु का उत्पादन करने वाला एक व्यक्ति अथवा एक फ़र्म होने कारण इसके उद्योग भी कहा जाता है। कोई अन्य फ़र्म इस उद्योग में शामिल नहीं हो सकती तथा एक फ़र्म होने कारण इसको छोड़ने का प्रश्न ही उत्पन्न नहीं होता।
6. कीमत विभेद (Price Discrimination) एकाधिकारी अपनी वस्तु की कीमत विभिन्न ग्राहकों से अलग-अलग प्राप्त कर सकता है। एकाधिकारी ही कीमत विभेद कर सकता है।
7. मांग वक्र (Demand Curve)-एकाधिकारी का कीमत पर नियन्त्रण होता है, परन्तु यदि एकाधिकारी कीमत अधिक रखता है तो मांग कम होगी। मांग में वृद्धि करने के लिए उसको कीमत घटानी पड़ती है। इसलिए मांग वक्र ऋणात्मक ढाल वाली होती है।
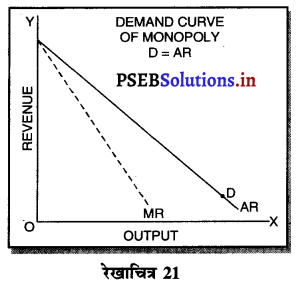
8. एकाधिकारी की संरचना (Formation of Monopoly)-एकाधिकारी बाज़ार
- सरकार से लाइसेंस लेकर
- वस्तु रजिस्टर्ड करवाकर
- व्यापारिक गुटबन्दी द्वारा
- प्राकृतिक साधनों पर नियन्त्रण द्वारा स्थापित की जाती है।
![]()
प्रश्न 3.
एकाधिकारी प्रतियोगिता से क्या अभिप्राय है ? एकाधिकारी प्रतियोगिता की विशेषताओं का वर्णन करो।
(What is Monopolistic Competition? Explain the characteristics of Monopolistic Competition.)
उत्तर-
एकाधिकारी प्रतियोगिता एक ऐसा बाज़ार है, जिसमें वस्तु को बेचने वालों की संख्या अधिक होती है, परन्तु प्रत्येक उत्पादक अपनी वस्तु को दूसरे उत्पादकों से विभिन्न बनाकर बेचने का प्रयत्न करता है। वस्तु भिन्नता (Product differention) इस बाज़ार की विशेष विशेषता होती है। वास्तविक जीवन में एकाधिकारी प्रतियोगिता की स्थिति पाई जाती है। यदि हम वास्तविक जीवन में देखते हैं तो प्रत्येक उत्पादक अपनी वस्तु का नाम रजिस्टर्ड करवा लेता है।
उस नाम पर कोई अन्य उत्पादन वस्तु की उत्पादकता नहीं कर सकता जैसे कि टैक्सला टेलीविज़न रजिस्टर्ड नाम है, परन्तु बाजार में अन्य टेलीविज़न जैसे कि बी० पी० एल०, ओनीडा, एल० जी०, अकाई, थामसन, फिलिप्स इत्यादि की प्रतियोगिता भी होती है। इसलिए इस बाज़ार में एक ओर एकाधिकारी पाई जाती है तथा दूसरी ओर प्रतियोगिता पाई जाती है। जिस कारण इस बाज़ार को एकाधिकारी प्रतियोगिता का बाज़ार कहा जाता है।
एकाधिकारी प्रतियोगिता की विशेषताएं (Characteristics of Monopolistic Competition) –
एकाधिकारी, प्रतियोगिता के बाज़ार की मुख्य विशेषताएं निम्नलिखित हैं-
1. बेचने वालों की अधिक संख्या (Large Number of Sellers)—इस बाज़ार में फ़र्मों की संख्या बहुत अधिक होती है। यह फ़में एक-दूसरे का मुकाबला करती हैं। बाज़ार में प्रत्येक फ़र्म का आकार सीमित होता है।
2. खरीदने वालों की अधिक संख्या (Large Number of buyers)-एकाधिकारी प्रतियोगिता में खरीदने वालों की संख्या बहुत अधिक होती है। प्रत्येक खरीदने वाला वस्तु की कीमत पर वस्तु के गुणों को ध्यान में रखकर इसकी खरीद करता है।
3. वस्तुओं में भिन्नता (Product Differentiation)-इस बाज़ार की सबसे महत्त्वपूर्ण विशेषता वस्तुओं में भिन्नता होती है, प्रत्येक उत्पादक अपनी वस्तु को हटकर बनाने का प्रयत्न करता है। इसलिए वस्तुओं का उत्पादन करते समय इनके रंग रूप, गुण आकार, पैकिंग, नाम इत्यादि में अन्तर पाकर वस्तु को अलग बनाने का प्रयत्न किया जाता है। यह वस्तुएं एक-दूसरे की नज़दीकी स्थानापन्न होती है। जैसे कि बाज़ार में टैक्सला, बी० पी० एल०, ओनीडा, फिलिप्स, एल० जी० इत्यादि टेलीविज़न एक-दूसरे के स्थानापन्न हैं।
4. बाज़ार में प्रवेश करने तथा छोड़ने की स्वतन्त्रता (Freedom of entry and exist)-एकाधिकारी प्रतियोगिता में फ़र्मे बाज़ार में जब चाहे प्रवेश कर सकती है अथवा फ़र्मे बाज़ार को छोड़कर कोई अन्य कार्य भी कर सकती हैं। इस बाज़ार में प्रवेश करना बहुत कठिन होता है, क्योंकि कार्य आरम्भ करने से पहले वस्तु का नाम इत्यादि रजिस्टर्ड करवाने पड़ते हैं तथा सरकार से आज्ञा लेकर कार्य आरम्भ किया जाता है। परन्तु हानि होने की स्थिति में फ़र्म उत्पादन बन्द कर सकती है।
5. बिक्री लागतें (Selling Costs)-बिक्री लागतों को प्रचार की लागतें भी कहा जाता है। प्रत्येक उत्पादक अपनी वस्तु की बिक्री को बढ़ाने के लिए वस्तु का प्रचार करता है। इसका मुख्य उद्देश्य ग्राहकों को वस्तु सम्बन्धी जानकारी देना होता है, ताकि लोग अधिक से अधिक वस्तु की खरीद करें। इस उद्देश्य के लिए अखबार, रसाले, टेलीविज़न, रेडियो, सिनेमा इत्यादि द्वारा अपनी वस्तु के गुण बताकर मनचाहे फिल्मी कलाकारों से प्रचार करवाया जाता है ताकि वस्तु की अधिक बिक्री हो सके।
6. अपूर्ण ज्ञान (Imperfect Knowledge)- अपूर्ण प्रतियोगिता में खरीददारों को वस्तु की कीमत वस्तु के गुणों तथा प्रकार के प्रति पूर्ण ज्ञान नहीं होता। सभी बाज़ार में बिकने वाली वस्तुओं में इतनी समानता होती है कि वस्तु सम्बन्धी पूर्ण जानकारी प्राप्त करनी असम्भव नहीं होती। इसलिए प्रचार, रीति-रिवाज, फैशन इत्यादि तत्त्वों से प्रभावित होकर वस्तु की खरीद की जाती है।
7. अपूर्ण गतिशीलता (Imperfect Mobility) -अपूर्ण प्रतियोगिता में उत्पादन के साधनों में पूर्ण गतिशीलता नहीं होती अर्थात् इनको एक स्थान से दूसरे स्थान तक ले जाना सम्भाव नहीं होता। विशेष तौर पर मजदूरों में कम गतिशीलता पाई जाती है, बोली, खान-पीन, पहरावा इत्यादि तत्त्व गतिशीलता के मार्ग में रुकावट बन जाते हैं।
8. कीमत प्रतियोगिता का अभाव (Absence of Price Competition)-इस बाज़ार में विशेष तौर पर फ़र्मों में कीमत प्रतियोगिता नहीं होती, क्योंकि फ़र्मों को यह ज्ञात होता है कि कीमत घटाने से न केवल विपक्षीय फ़र्मों को ही नुकसान होगा, बल्कि उनको स्वयं भी हानि सहन करनी पड़ेगी। इसलिए लाभ बढ़ाने के लिए फ़र्मे गुटबन्दी बना लेती हैं।
प्रश्न 4.
पूर्ण प्रतियोगिता, एकाधिकारी तथा एकाधिकारी प्रतियोगिता में अन्तर बताओ। (Explain the difference between Perfect Competition, Monopoly and Monopolistic Competition.)
उत्तर-
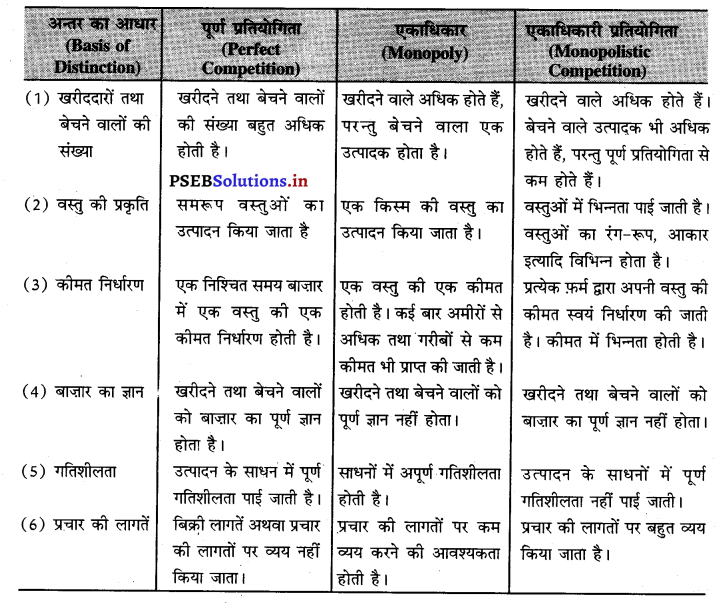
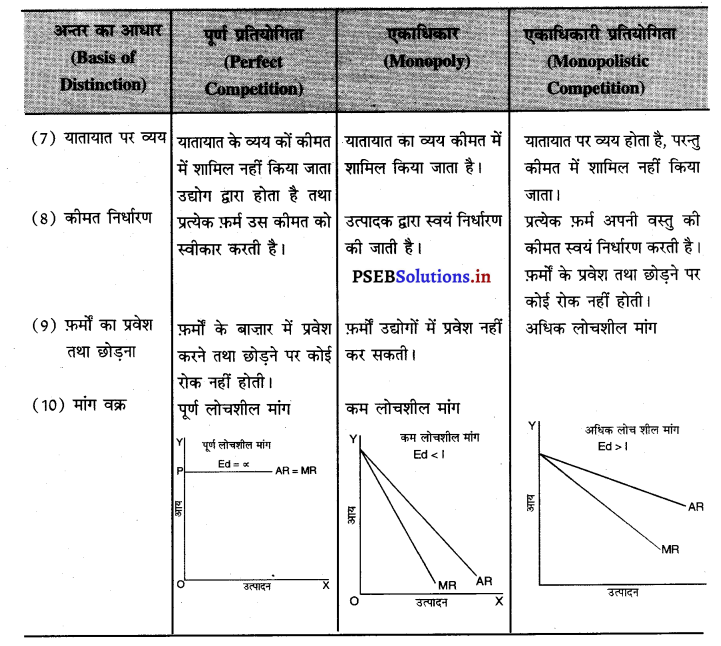
इनमें अन्तर को निम्नलिखित अनुसार स्पष्ट किया जा सकता है-
![]()
(D) अल्प अधिकार (Oligopoly)
प्रश्न 5.
अल्प-अधिकार से क्या अभिप्राय है ? इसकी विशेषताएं बताएं। (What is meant by Oligopoly ? Explain the main features of Oligopoly.)
उत्तर-
अल्प-अधिकार आज कल के युग में एक व्यावहारिक बाज़ार है। इसमें कम फर्मे (A few Firms) होती हैं। यह फर्मे एक समान वस्तुओं का उत्पादन करती हैं जैसे कि सीमेंट, आटा आदि अथवा एक दूसरे के नज़दीक की वस्तुओं का उत्पादन करती हैं। इस बाज़ार में विक्रेताओं की संख्या 2 से 10 तक होती है जो विलियम फैलनर के अनुसार, “अल्प-अधिकार कम फर्मों में प्रतियोगिता का बाज़ार होता है।” (Oligopoly is a market with competition among the few. -Fellner)
अल्प-अधिकार की विशेषताएं (Features of Oligopoly) –
- आत्मनिर्भरता (Independence)-इस बाज़ार में फर्मे एक-दूसरे पर निर्भर होती हैं। एक फर्म का निर्णय दूसरी फर्मों को प्रभावित करता है। यदि एक फर्म कीमत कम कर देती है तो दूसरी फर्म को भी कीमत कम करनी पड़ती
- प्रचार (Advertisement)-इस बाज़ार में प्रचार पर बहुत व्यय किया जाता है। प्रचार करके प्रत्येक उत्पादक अधिक बिक्री करने का यत्न करता है।
- प्रतियोगिता (Competition)- इस बाज़ार में फर्मों में प्रतियोगिता पाई जाती है। यदि एक उत्पादक वस्तु के साथ कोई तोहफा देता है तो दूसरे उत्पादकों को भी ऐसा ही करना पड़ता है।
- मांग वक्र (Demand Curve)-इस बज़ार में मांग वक्र स्पष्ट नहीं होती। एक फर्म द्वारा कीमत कम अथवा अधिक करने से दूसरी फर्मे भी वैसा ही करती हैं। टेढ़ी मांग वक्र इसलिए माँग वक्र टेढ़ी रेखा होती है।
- एकसारता का अभाव (No Uniformity)प्रत्येक फर्म का आकार अलग होता है। एक फर्म बढ़ी तथा दूसरी फर्मे छोटे आकार की हो सकती हैं।
- समूह व्यवहार (Group Behaviour)-प्रत्येक फर्म का अपना विशेष बाज़ार होता है। नई फमें आ सकती हैं। परन्तु जो फर्मे लगी होती हैं वह आसानी से फर्मों को आने नहीं देतीं।