Punjab State Board PSEB 12th Class Chemistry Book Solutions Chapter 8 The d-and f-Block Elements Textbook Exercise Questions and Answers.
PSEB Solutions for Class 12 Chemistry Chapter 8 The d-and f-Block Elements
PSEB 12th Class Chemistry Guide The d-and f-Block Elements InText Questions and Answers
Question 1.
Write down the electronic configuration of:
(i) Cr3+
(ii) Cu+
(iii) Co2+
(iv) Mn2+
(v) Pm3+
(vi) Ce4+
(vii) Lu2+
(viii) Th4+
Answer:
(i)Cr3+ = [Ar] 3d3
(ii) Cu+ = [Ar] 3d10
(iii) Co2+ = [Ar] 3d7
(iv) Mn2+ = [Ar] 3d5
(v) Pm3+ = [Xe] 4f4
(vi) Ce4+ = [Xe]
(vii) Lu2+ = [Xe] 4f145d1
(viii) Th4+ = [Rn]
Question 2.
Why are Mn2+ compounds more stable than Fe2+ towards oxidation to their + 3 state?
Answer:
Electronic configuration of Mn2+ is [Ar]18 3d5
Electronic configuration of Fe2+ is [Ar]18 3d6
It is known that half-filled and fully-filled orbitals are more stable. Therefore, Mn in (+ 2) state has a stable d5 configuration. This is the reason Mn2+ shows resistance to oxidation to Mn3+. Also, Fe2+ has 3d6 configuration and by losing one electron, its configuration changes to a more stable 3d5 configuration. Therefore, Fe2+ easily gets oxidised to Fe3+ oxidation state.
Question 3.
Explain briefly how +2 state becomes more and more stable in the first half of the first row transition elements with increasing atomic number?
Answer:
As the atomic number increases from 21 to 25, the number of electrons in the 3d-orbital also increases from 1 to 5. +2 oxidation state is attained by the loss of the two 4s electrons by these metals. Sc does not exhibit +2 oxidation state. As the number of d-electrons in +2 state increases from Ti to Mn, the stability of +2 state increases (d-orbital gradually becoming half filled). Mn(+2) has d5 electrons which is highly stable.
![]()
Question 4.
To what extent do the electronic configurations decide the stability of oxidation states in the first series of the transition elements? Illustrate your answer with examples.
Answer:
The stability of oxidation states in the first series of the first transition elements are related to their electronic configurations.
The first five elements of the first transition series upto Mn in which the 3d-subshell is not more than half-filled, the minimum oxidation state is given by fife number of electrons in the outer s-subshell and the maximum oxidation state is given by the sum of the outer s and d-electrons. For example, Sc does not show +2 oxidation state. Its electronic configuration is 4s2 3d1. It loses all the three electrons to form SC3+.+3 oxidation state is very stable as by losing all three electrons, it attains the stable configuration of Argon. For Mn, +2 oxidation state is very stable, as after losing two 4s electrons, the d-orbitals become half-filled.
Question 5.
What may be the stable oxidation state of the transition elements with the following d-electron configurations in the ground state of their atoms?
3d3, 3d5, 3d8 and 3d4
Answer:
Stable oxidation states:
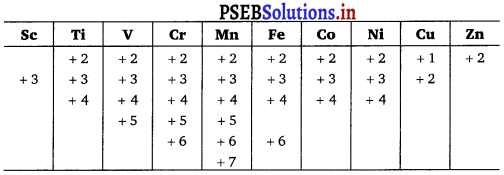
3d3 (vanadium): +2, +3, +4, +5
3d5 (chromium): +3, +4, +6
3d5 (manganese): +2, +4, +6, +7
3d8 (cobalt) : +2, +3 (in complexes)
3d4 : There is no d4 configuration in the ground state.
Question 6.
Name the oxometal anions of the first series of the transition metals in which the metal exhibits the oxidation state equal to its group number.
Answer:
(i) Vanadate, \(\mathrm{VO}_{3}^{-}\)
Oxidation state of V is + 5.
(ii) Chromate, \(\mathrm{CrO}_{4}^{2-}\)
Oxidation state of Cr is + 6.
(iii) Permanganate, \(\mathrm{MnO}_{4}^{-}\)
Oxidation state of Mn is + 7.
![]()
Question 7.
What is lanthanoid contraction? What are the consequences of lanthanoid contraction?
Answer:
As we move along the lanthanoid series, the atomic number increases gradually by one. This means that the number of electrons and protons present in an atom also increases by one. As electrons are being added to the same shell, the effective nuclear charge increases. This happens because the increase in nuclear attraction due to the addition of proton is more pronounced than the increase in the interelectronic repulsions due to the addition of electron. Also, with the increase in atomic number, the number of electrons in the 4f orbital also increases. The 4f electrons have poor shielding effect. Therefore, the effective nuclear charge experienced by the outer electrons increases. Consequently, the attraction of the nucleus for the outermost electrons increases. This results in a steady decrease in the size of lanthanoids with the increase in the atomic number. This is termed as lanthanoid contraction.
Consequences of lanthanoid contraction
- There is similarity in the properties of second and third transition series.
- Separation of lanthanoids is possible due to lanthanoid contraction.
- It is due to lanthanoid contraction that there is variation in the basic strength of lanthanoid hydroxides. (Basic strength decreases from La(OH)3 to Lu(OH)3.)
Question 8.
What are the characteristics of the transition elements and why are they called transition elements? Which of the d-block elements may not he regarded as the transition eledaents?
Answer:
Characteristics of the Transition Elements (d-Block)
1. Electronic configuration : General electronic configuration of these elements is (n -1) d1-10 ns1-2.
2. Physical properties : These elements have metallic properties such as metallic lustre, high tensile strength, ductility, malleability, high thermal and electrical conductivity, low volatility (except Zn, Cd, Hg), hardness, etc. Their melting points are high.
3. Atomic and ionic size : In a given series, there is a progressive decrease in radius with increasing atomic number.
4. Ionisation enthalpies : Due to an increase in nuclear charge which accompanies the filling of inner d-orbitals, there is an increase in ionisation enthalpy along each series of the transition elements from left to right.
5. Oxidation states : These elements exhibit variable oxidation states.
e.g.,1 Transition series:
6. Trends in M2+/M⊖ values: The general trend towards less negative E0 values across the series is related to the general increase in the sum of the first and second ionisation enthalpies.
7. Trends in M3+/M2+E⊖ values : Low value of Ee shows the stability of ion (either d5 or d10 configuration).
8. Magnetic properties : These elements show diamagnetism and paramagnetism.
9. Formation of coloured salts : The compounds of transition elements form coloured ions, e.g., Mn3+ violet; Fe2+ green, etc.
10. Complex formation : These elements form complex compounds due to their small size and high charge density, e.g., [PtCl4]2-
11. Catalyst: Many of these elements are used as catalyst e.g., V2O5 is used as a catalyst in contact process for the manufacture of H2SO4.
12. Interstitial compound formation : Transition elements form interstitial compounds. It means the compounds in which H, C or N etc. are trapped inside the crystal lattices of metals.
13. Alloy formation : Because of similar radii and other characteristics alloys are readily formed by these metals.
The d-block elements are called transition elements because these elements represent change or transition in properties from s-block to p-block elements.
The electronic configuration of Zn, Cd and Hg is represented by the general formula (n – 1)d10ns2. These elements have completely filled d-orbitals in ground state as well as in their common oxidation states. Therefore, they may not be regarded as the transition elements.
Question 9.
In what way is the electronic configuration of the transition elements different from that of the non-transition elements?
Answer:
Transition elements contain incompletely filled d-subshell, Le., their electronic configuration is (n – 1)d1-10ns1-2 whereas non-transition elements have no d-subshell or their subshell is completely filled and have ns1-2 or ns2np1-6 in their outermost shell.
![]()
Question 10.
What are the different oxidation states exhibited by the lanthanoids?
Answer:
In the lanthanoid series, + 3 oxidation state is most common i.e., Ln(III) compounds are predominant. However, + 2 and + 4 oxidation states can also be found in the solution or in solid compounds.
Question 11.
Explain giving reasons:
(i) Transition metals and many of their compounds show paramagnetic behaviour.
(ii) The enthalpies of atomisation of the transition metals are high.
(iii) The transition metals generally form coloured compounds.
(iv) Transition metals and their many compounds act as good catalyst.
Answer:
(i) Transition metals and many of their compounds show paramagnetic behaviour. Paramagnetism arises due to the presence of unpaired electrons with each electron having a magnetic moment associated with its spin angular momentum and orbital angular momentum. However, in the first transition series, the orbital angular momentum is quenched. Therefore, the resulting paramagnetism is only because of the unpaired electron.
(ii) Transition metals have high effective nuclear charge and a large number of valence electrons. Therefore, they form very strong metallic bonds. As a result, the enthalpy of atomisation of transition metals is high.
(iii) Formation of coloured compounds by transition metals is due to partial adsorption of visible light. The electron absorbs the radiation of a particular frequency (of visible region) and jumps into next orbital.
(iv) Catalysts, at the solid surface, involve the formation of bonds between reactants molecules and atoms of the surface of the catalyst (I row transition metals utilised 3d and 4s-electrons for bonding). This has the effect of increasing the concentration of the reactants at the catalyst surface and also lowering of the activation energy.
Transition metal ions show variable oxidation states so they are effective catalysts, e.g.
![]()
Mechanism of catalysing action of Fe3+ in the above reaction
(a) 2Fe3+ + 2I– → 2Fe2+ + I2
(b) 2Fe2+ + \(\mathrm{S}_{2} \mathrm{O}_{8}^{2-}\) → 2Fe3+ + \(2 \mathrm{SO}_{4}^{2-}\)
Question 12.
What are interstitial compounds? Why are such compounds well known for transition metals?
Answer:
Interstitial compounds are those in which small atoms occupy the interstitial sites in the crystal lattice. Interstitial compounds are well known for transition metals because small-sized atoms of H, B, C, N, etc., can easily occupy position in the voids present in the crystal lattices of transition metals.
![]()
Question 13.
How is the variability in oxidation states of transition metals different from that of the non-transition metals? Illustrate with examples.
Answer:
The oxidation states of transition elements differ from each other by unity e.g., Fe2+ and Fe3+, Cu+ and Cu2+ (due to incomplete filling of d-orbitals) whereas oxidation states of non-transition elements normally differ by two units e.g., Pb2+ and Pb4+, Sn2+ and Sn4+ etc.
Question 14.
Describe the preparation of potassium dichromate from iron chromite ore. What is the effect of increasing pH of a solution of potassium dichromate?
Answer:
Potassium dichromate is prepared from chromite ore FeCr2O4 as follows :
Step I: Conversion of chromite ore to sodium chromate.
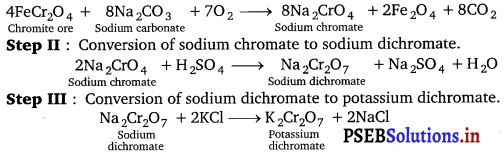
Effect of pH : The chromates and dichromates can interconverted to each other in aqueous solution by increasing the pH of the solution.
\(\mathrm{CrO}_{4}^{2-}\) + 2OH– ⇌ \(2 \mathrm{CrO}_{4}^{2-}\) + H2O
Question 15.
Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with
(i) iodide
(ii) iron (H) solution and
(iii) H2S.
Answer:
(i) \(\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}\) + 14H+ + 6I– → 2Cr3+ + 7H2O + 3I2
(ii) \(\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}\) + 14H+ + 6Fe2+ → 2Cr3+ + 7H2O + 6Fe3+
(iii) \(\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}\) + 8H+ + 3H2S → Cr3+ + 7H2O + 3S
![]()
Question 16.
Describe the preparation of potassium permanganate. How does the acidified permanganate solution reacts with
(i) iron
(ii) ions
(iii) SO2 and
(iii) oxalic acid? Write the ionic equations for the reactions.
Answer:
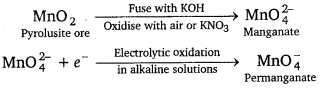
Preparation of potassium permanganate, KMnO4: Potassium permanganate is prepared by the fusion of MnO2 (pyrolusite) with potassium hydroxide and an oxidising agent such as KNO3 to form potassium manganate which disproportionate in a neutral or acidic solution to form permanganate.
2MnO2 + 4KOH + O2 → 2K2MnO4 + H2O
\(3 \mathrm{MnO}_{4}^{2-}\) + 4H+ → \(\mathrm{MnO}_{4}^{-}\) + MnO2 + H2O
On large scale : It is prepared by the alkaline oxidative fusion of MnO2 to form potassium manganate. The electrolytic oxidation of potassium manganate in alkaline solution produces KMnO4, at the anode.
Reaction of KMnO4 in acidic medium
(a) Oxidises iron (II) ions to iron (III) ions.
\(\mathrm{MnO}_{4}^{-}\) + 5Fe2+ + 8H2+ → Mn2+ + 5Fe3+ + 4H2O
(b) Oxidises SO2 to sulphuric acid.
\(2 \mathrm{MnO}_{4}^{-}\) + 5SO2 + 2H2O → 2Mn2+ + 5SO2 + 4H2O
(c) Oxidises oxalic acid to carbon dioxide.
\(2 \mathrm{MnO}_{4}^{-}\) + \(5 \mathrm{C}_{2} \mathrm{O}_{4}^{2-}\) + 16H+ → 2Mn2+ + 10CO2 + 8H2O
Question 17.
For M2+/M and M3+/M2+ systems, the E⊖ values for some metals are as follows:
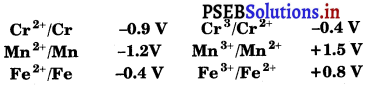
Use this data to comment upon :
(i) the stability of Fe3+ in acid solution as compared to that of Cr3+ or Mn3+ and
(ii) the ease with which iron can be oxidised as compared to a similar process for either chromium or manganese metal.
Answer:
(i) Higher the reduction potential of a species, greater is the tendency for its reduction to take place. Therefore, Mn3+ with highest reduction potential would be readily reduced to Mn2+ and hence is the least stable.
Thus, from the value of reduction potential, it is clear that the stability of Fe3+ in acidic solution is more than Mn3+ but less than that of Cr3+.
(ii) Lower the reduction potential or higher the oxidation potential of a species, greater the ease with which its oxidation will take place. Thus, order of tendency to undergo oxidation is Fe < Cr < Mn.
Question 18.
Predict which of the following will be coloured in aqueous solution?
Ti3+, V3+, Cu+, Sc3+, Mn2+, Fe3+ and Co2+. Give reasons for each.
Answer:
Only those ions will be coloured which have incompletely filled d-orbitals. Those with fully-filled or empty d-orbitals are colourless. Due to d-d transition, Ti3+, V3+, Mn2+, Fe3+ and Co2+ are coloured.
![]()
Question 19.
Compare the stability of + 2 oxidation state for the elements of the first transition series.
Answer:
The decreasing negative electrode potentials of M2+/M+1 in the first transition series shows that in general, the stability of +2 oxidation state decrease from left-to right (exception being Mn and Zn). The decrease in the negative electrode potentials is due to increase in the sum IE2 + IE2.
Question 20.
Compare the chemistry of actinoids with that of the lanthanoids with special reference to :
(i) electronic configuration
(ii) oxidation states
(iii) atomic and ionic sizes and
(iv) chemical reactivity.
Answer:
(i) Electronic configuration : The general electronic configuration of lanthanoids is [Xe]54 4f1-14 5d0-16s2 whereas that of actinoids is [Rn]865f1-146d0-17s2. Thus, lanthanoids belong to 4/-series whereas actinoids belong to 5/-series.
(ii) Oxidation states : Lanthanoids show limited oxidation states (+2, +3, +4), out of which, +3 is most common. This is because of a large energy gap between 4/, 5d and 6s subshells. On the other hand, actinoids show a large number of oxidation states because of small energy gap between 5/, 6d and 7s subshells.
(iii) Atomic and ionic sizes : Both show decrease in size of their atoms or ions in +3 oxidation state. In lanthanoids, the decrease is called lanthanoid contraction whereas in actinoids, it is called actinoid contraction. However, the contraction is greater from element to element in actinoids due to poorer shielding by 5/-electrons.
(iv) Chemical reactivity : In general, the earlier members of the lanthanoid series are quite reactive (similar to calcium) but with increasing atomic number, they behave more like aluminium.
Values for E⊖ for the half-reaction :
Ln3+ (aq) + 3e– → Ln(s)
are in the range of -2.2 to -2.4 V except for Eu for which the value is -2.0 V. This is of course, a small variation.
The metals combine with hydrogen when gently heated in the gas. The carbides, Ln3C , Ln2C3 and LnC2 are formed when the metals are heated with carbon. They liberate hydrogen from dilute acids and bum in halogens to form halides. They form oxides and hydroxides —M2O3 and M(OH)3. The hydroxides are definite compounds, not just hydrated oxides, basic like alkaline earth metal oxides and hydroxides.
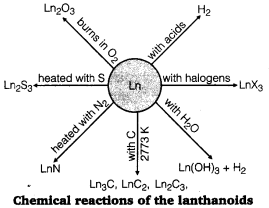
The actinoids are highly reactive metals, especially when finely divided. For example, the action of boiling water on them, gives a mixture of oxide and hydride and combination with most non-metals takes place at moderate temperatures. Hydrochloric acid attacks all metals but most are slightly affected by nitric acid owing to the formation of protective oxide layers; alkalis have no action.
![]()
Question 21.
How would you account for the following:
(i) Of the d4 species, Cr2+ is strongly reducing while manganese (III) is strongly oxidising.
(ii) Cobalt (II) is stable in aqueous solution but in the presence of complexing reagents, it is easily oxidised.
(iii) The d1 configuration is very unstable in ions.
Answer:
(i) E⊖ value for Cr3+ /Cr2+ is negative (-0.41 V) whereas E⊖ value for Mn3+/Mn2+ is positive (+1.57 V). Thus, Cr2+ ions can easily undergo oxidation to give Cr3+ ions and, therefore, act as strong reducing agent. On the other hand, Mn3+ can easily undergo reduction to give Mn2+ and hence act as oxidising agent.
(ii) Co(III) has greater tendency to form coordination complexes than Co (II). Thus, in the presence of ligands, Co (II) charges to Co (III), i.e., it easily oxidised.
(iii) The ions with d1 configuration have the tendency to lose the only electron present in d-subshell to acquire stable d0 configuration. Therefore, they are unstable and undergo oxidation or disproportionation.
Question 22.
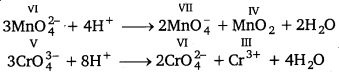
What is meant by ‘disproportionation’? Give two examples of disproportionation reaction in aqueous solution.
Answer:
Disproportionation reactions are those reactions in which the same substance undergoes oxidation as well as reduction. In disproportion reaction, oxidation number of an element increases as well as decreases to form two different products, e.g.,
Question 23.
Which metal in the first series of transition metals exhibits +1 oxidation state most frequently and why?
Answer:
Copper has electronic configuration 3d10 4s1 will attain a completely filled d-orbital and a stable configuration on losing 4s1 electron i.e., [Ar] 3d10.
![]()
Question 24.
Calculate the number of unpaired electrons in the following gaseous ions: Mn3+, Cr3+, V3+ and Ti3+. Which one of these is the most stable in aqueous solution?
Answer:
Mn3+ = 3d4 = 4 unpaired electrons
Cr3+ = 3d3 = 3 unpaired electrons
V3+ = 3d2 = 2 unpaired electrons
Ti3+ = 3d1 =1 unpaired electron
Out of these species Cr3+ is the most stable in aqueous solution due to its tendency of complex formation.
Question 25.
Give examples and suggest reasons for the following features of the transition metal chemistry :
(i) The lowest oxide of transition metal is basic, the highest is amphoteric/acidic.
(ii) A transition metal exhibits higher oxidation state in oxides and fluorides.
(iii) The highest oxidation state is exhibited in oxo-anions of a metal.
Answer:
(i) The lowest oxide of transition metal is basic because the metal atom has low oxidation state. This means that it can donate valence electrons which are not involved in bonding to act like a base. Whereas the highest oxide is amphoteric/acidic due to the highest oxidation state as the valence electrons are involved in bonding and are unavailable. For example, MnO is basic whereas Mn2O7 is acidic.
(ii) A transition metal exhibits higher oxidation states in oxides and fluorides because oxygen and fluorine are highly electronegative elements, small in size (and strongest oxidising agents). For example, osmium shows an oxidation state of +6 in O2F6 and vanadium shows an oxidation state of +5 in V2O5.
(iii) Oxometal anions have the highest oxidation state, e.g., Cr in \(\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}\) has an oxidation state of +6 whereas Mn in \(\mathrm{MnO}_{4}^{-}\) has an oxidation state of +7. This is again due to the combination of the metal with oxygen, which is highly electronegative and oxidising element.
Question 26.
Indicate the steps in the preparation of:
(i) K2Cr2O7 from chromite ore.
(ii) KMnO4 from pyrolusite ore.
Answer:
(i) Potassium dichromate is prepared from chromite ore FeCr2O4 as follows :
Step I: Conversion of chromite ore to sodium chromate.

Effect of pH : The chromates and dichromates can interconverted to each other in aqueous solution by increasing the pH of the solution.
\(\mathrm{CrO}_{4}^{2-}\) + 2OH– ⇌ \(2 \mathrm{CrO}_{4}^{2-}\) + H2O
(ii) Preparation of potassium permanganate, KMnO4: Potassium permanganate is prepared by the fusion of MnO2 (pyrolusite) with potassium hydroxide and an oxidising agent such as KNO3 to form potassium manganate which disproportionate in a neutral or acidic solution to form permanganate.
2MnO2 + 4KOH + O2 → 2K2MnO4 + H2O
\(3 \mathrm{MnO}_{4}^{2-}\) + 4H+ → \(\mathrm{MnO}_{4}^{-}\) + MnO2 + H2O
On large scale : It is prepared by the alkaline oxidative fusion of MnO2 to form potassium manganate. The electrolytic oxidation of potassium manganate in alkaline solution produces KMnO4, at the anode.

Reaction of KMnO4 in acidic medium
(a) Oxidises iron (II) ions to iron (III) ions.
\(\mathrm{MnO}_{4}^{-}\) + 5Fe2+ + 8H2+ → Mn2+ + 5Fe3+ + 4H2O
(b) Oxidises SO2 to sulphuric acid.
\(2 \mathrm{MnO}_{4}^{-}\) + 5SO2 + 2H2O → 2Mn2+ + 5SO2 + 4H2O
(c) Oxidises oxalic acid to carbon dioxide.
\(2 \mathrm{MnO}_{4}^{-}\) + \(5 \mathrm{C}_{2} \mathrm{O}_{4}^{2-}\) + 16H+ → 2Mn2+ + 10CO2 + 8H2O
![]()
Question 27.
What are alloys? Name an important alloy which contains some of the lanthanoid metals. Mention its uses.
Answer:
An alloy is a homogeneous mixture of two or more metals, or metals and non-metals. An important alloy containing lanthanoid metals is misch metal which contains 95% lanthanoid metals and 5% iron along with traces of S, C, Ca and Al. It is used in Mg-based alloy to produce bullets, shells and lighter flints.
Question 28.
What are inner-transition elements? Decide which of the following atomic numbers are the atomic numbers of the inner-transition elements:
29, 59, 74, 95, 102, 104.
Answer:
The f-block elements, i.e., in which the last electron enters into /-subshell are called inner-transition elements. These include lanthanoids (58-71) and actinoids (90-103). Thus, elements with atomic numbers 59, 95 and 102 are inner-transition elements.
Question 29.
The chemistry of the actinoid elements is not so smooth as that of the lanthanoids. Justify this statement by giving some examples from the oxidation state of these elements.
Answer:
Lanthanoids show a limited number of oxidation state, viz., +2, +3 and +4 (out of which +3 is the most common). This is because of a large energy gap between 4/, 5d and 6s subshells. The dominant oxidation state of actinoids is also +3 but they show a number of other oxidation states also, e.g., uranium(Z = 92) and plutonium (Z = 94), show +3, +4, +5 and +6, neptunium (Z = 94) shows +3, +4, +5 and +7, etc. This is due to small energy difference between 5/, 6 d and 7s subshells of the actinoids.
![]()
Question 30.
Which is the last element in the series of the actinoids? Write the electronic configuration of this element. Comment on the possible oxidation state of this element.
Answer:
The last element in the actinoid series is lawrencium (Lr). Its atomic number is 103 and its electronic configuration is [Rn]5f14 6d1 7s2. The most common oxidation state displayed by it is + 3; because after losing 3 electrons it attains stable f14 configuration.
Question 31.
Use Hund’s rule to derive the electronic configuration of Ce3+ ion and calculate its magnetic moment on the basis of spin only formula.
Answer:
58Ce =[Xe]54f15d16s2
Ce3+ = [Xe]544f1, i.e., there is only one unpaired electron, i.e., n = 1. Hence, p = \(\sqrt{n(n+2)}\) = \(\sqrt{1(1+2)}\) = \(\sqrt{3}\) = 1.73 BM
Question 32.
Name the members of the lanthanoid series which exhibit +4 oxidation states and those which exhibit +2 oxidation states. Try to correlate this type of behaviour with the electronic configurations of these elements.
Answer:
+4 = 58Ce , 59Pr, 60Nd, 65Tb, 66Dy
+2 = 60Nd, 62Su 63Eu, 69Tm, 70Yb
+2 oxidation state is exhibited when the lanthanoid has the configuration 5d06s2, so that 2 electrons are easily lost. +4 oxidation state is exhibited when the configuration left is close to 4f0 (e.g., 4f0, 4f1, 4f2) or close to 4f7 (e.g., 4f7or 4f8)
![]()
Question 33.
Compare the chemistry of the actinoids with that of lanthanoids with reference to:
(i) electronic configuration
(ii) oxidation states and
(iii) chemical reactivity.
Answer:
| Characteristics | Lanthanoids | Actinoids |
| (i) Electronic configuration | [Xe] 4/1-145d0-16s2 | [Rn] 5/1“14 6d0_1 7s2 |
| (ii) Oxidation states | Besides +3 oxidation state lanthanoids show +2 and +4 oxidation state only in a few cases. | Besides +3 oxidation state, actinoids show higher oxidation state of +4, +5, +6, +7 also because of smaller energy gap between 5/, 6d and 7s subshell. |
| (iv) General chemical reactivity elements | These are less reactive metals.
Lesser tendency towards complex formation. Do not form oxacation. Compounds are less basic. |
These are highly reactive metals
Greater tendency towards complex formation. Form oxocation.Compounds are more basic. |
Question 34.
Write the electronic configurations of the elements with the atomic numbers 61, 91,101 and 109.
Answer:
Z = 61 (Promethium, Pm), electronic configuration [Xe] 4f55d06s2
Z = 91 (Protactium, Pa), electronic configuration = [Rn] 5f26d1s2
Z = 101 (Mendelevium, Md), electronic configuration = [Rn] 5f136d07s2
Z =109 (Meitnerium, Mt), electronic configuration = [Rn] 5f146d7 7s2
Question 35.
Compare the general characteristics of the first series of the transition metals with those of the second and third series metals in the respective vertical columns. Give special emphasis on the following points :
(i) Electronic configurations,
(ii) Oxidation states,
(iii) Ionisation enthalpies and
(iv) Atomic sizes
Answer:
(i) Electronic configurations : The elements in the same vertical column generally have similar electronic configurations. Although the first series shows only two exceptions, i.e., Cr = 3d54s1 and Cu = 3d104s1 but the second series shows more exceptions, e.g., Mo(42) = 4ds5s1,Tc(43) = 4d65s1,Ru(44) = 4d75s1 Rh(45) = 4d85s1, Pb(46) = 4d105s0, Ag(47) = 4d105s1. Similarly, in the third series, W(74) = 5d46s2, Pt(78) = 5d96s1 and Au(79) = 5d10 6d1. Hence, in the same vertical column, in a number of cases, the electronic configuration of the three series are not similar.
(ii) Oxidation states : The elements in the same vertical column generally show similar oxidation states. The number of oxidation states shown by the elements in the middle of each series is maximum and minimum at the extreme ends.
(iii) Ionisation enthalpies : The first ionisation enthalpies in each series generally increase gradually as we move from left to right though some exceptions are observed in each series. The first ionisation enthalpies of some elements in the second (4d) series are higher while some of them have lower value than the elements of 3d series in the same vertical column. However, the first ionisation enthalpies of third (5d) series are higher than those of 3d and 4d series. This is because of weak shielding of nucleus of 4/-electrons in the 5d-series.
(iv) Atomic sizes : Generally, ions of the same charge or atoms in a given series show progressively decrease in radius with increasing atomic number though the decrease is quite small. But the size of the atoms of the 4d-series is larger than the corresponding elements of the 3d-series whereas those of corresponding elements of the 5d-series are nearly the same as those of 4d-series due to lanthanoid contraction.
![]()
Question 36.
Write down the number of 3d electrons in each of the following ions:
Ti2+, V2+, Cr3+, Mn2+, Fe2+, Fe3+, Co2+, Ni2+ and Cu2+. Indicate how would you expect the five 3d-orbitals to be occupied for these hydrated ions (octahedral).
Answer:
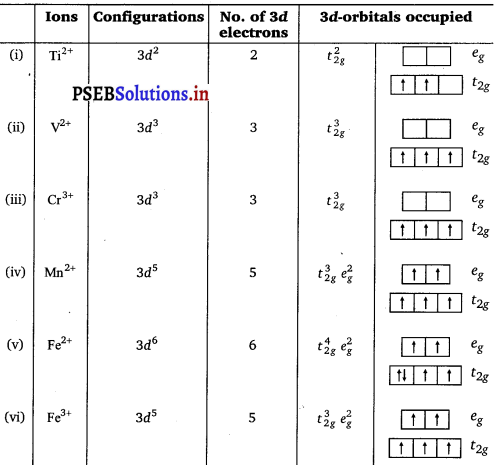
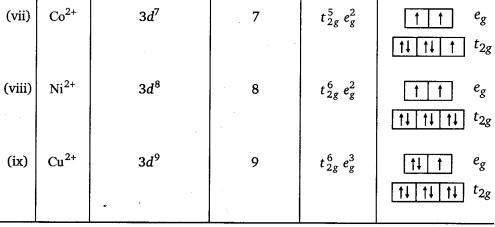
Question 37.
Comment on the statement that elements of the first transition series possess many properties different from those of heavier transition elements.
Answer:
The given statement is true. Some evidences in support of this statement are given below :
- Atomic radii of the heavier transition elements (4 d and 5d series) are larger than those of the corresponding elements of the first transition series though those of 4d and 5d series are very close to each other.
- Ionisation enthalpies of 5d series are higher than the corresponding elements of 3d and 4d series.
- Enthalpies of atomisation of 4d and 5d series are higher than the corresponding elements of first series.
- Melting and boiling points of heavier transition elements are greater than those of the first transition series due to stronger intermetallic bonding.
Question 38.
What can be inferred from the magnetic moment values of the following complex species?
| Example | Magnetic Moment (BM) |
| K4[Mn(CN)6] | 2.2 |
| [Fe(H2O)6]2+ | 5.3 |
| K2[Mncl4] | 5.9 |
Answer:
Magnetic moment (μ) = \(\sqrt{n(n+2)}\) BM
For n = 1, μ = \(\sqrt{1(1+2)}\) = \(\sqrt{3}\) = 1.73;
For n = 2, μ = \(\sqrt{2(2+2)}\) = \(\sqrt{8}\) = 2.83;
For n = 3, μ = \(\sqrt{3(3+2)}\) = \(\sqrt{15}\) = 3.87;
For n = 4, μ = \(\sqrt{4(4+2)}\) = \(\sqrt{24}\) = 4.90;
For n = 5, μ = \(\sqrt{5(5+2)}\) = \(\sqrt{35}\) = 5.92
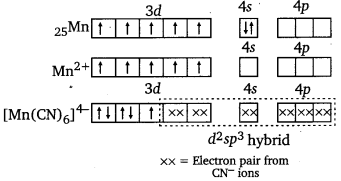
K4[Mn(CN)6]
Here, Mn is in +2 oxidation, state, i.e., as Mn2+. μ = 2.2 BM shows it has only one unpaired electron.
Hence, when CN– ligands approach Mn2+ion, the electrons in 3d pair up
Hence, CN– is a strong ligand. The hybridisation involved is d2sp3 forming inner orbital octahedral complex.
[Fe(H2O)6]2+
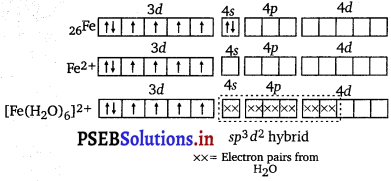
Here, Fe is in +2 oxidation state, i.e., as Fe2+. μ = 5.3 BM shows that there are four unpaired electrons. This means that the electrons in 3d do not pair up when the ligand H2O molecules approach. Hence, H2O is a weak ligand. To accommodate the electrons donated by six H20 molecules, the hybridisation will be sp3d2.
Hence, it will be an outer orbital octahedral complex.
K2[MnCl4]
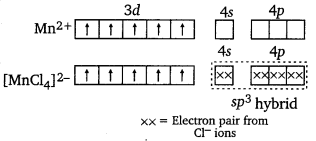
Here, Mn is in +2 oxidation state, i.e., as Mn2+. μ = 5.9 BM shows that there are five unpaired electrons. Hence, the hybridisation involved will be sp3 and the complex will be tetrahedral.
![]()
Chemistry Guide for Class 12 PSEB The d-and f-Block Elements Textbook Questions and Answers
Question 1.
Silver atom has completely filled d-orbitals (4d10) in its ground state. How can you say that it is a transition element?
Answer:
Silver (Z = 47) can show +2 oxidation state in which it has incompletely filled d-subshell (4d9 configuration). So, silver is a transition element.
Question 2.
In the series Sc (Z = 21) to Zn (Z = 30), the enthalpy of atomisation of zinc is the lowest i.e., 126 kJ mol-1. Why?
Answer:
In the first series of transition elements Sc to Zn, all elements have one or more unpaired electrons except zinc which has no unpaired electron as its outer electronic configuration is 3d104s2. Hence, interatomic metallic bonding (M-M bonding) is weaker in zinc. Therefore, enthalpy of atomisation is lowest.
Question 3.
Which of the 3d series of the transition metals exhibits the largest number of oxidation states and why?
Answer:
Mn (atomic number = 25) has electronic configuration[Ar] 3d5 4s2.
Mn has the maximum number of unpaffed electrons present in the d-subshell (5 electrons). Hence, Mn exhibits the largest number of oxidation states, i.e., + 2 to + 7 in its compounds.
![]()
Question 4.
The E⊖(M2+/M) value for copper is positive (+0.34 V). What is possible reason for this? (Hint: Consider its high △aH⊖ and low △hydH⊖)
Answer:
E (M2+/M) for any metal is related to the sum of the enthalpy change taking place in the following steps:
M(s) + △aH → M(g), (△aH = Enthalpy of atomisation)
M(g) + △iH → M2+(g) (△iH = Ionisation enthalpy)
M2+(g) + aq → M2+(aq) + △hydH
(△hydH = Hydration enthalpy)
Copper has high enthalpy of ionisation and relatively low enthalpy of hydration. So, E⊖(Cu2+/Cu) is positive. The high energy to transform Cu(s) to Cu2+(aq) is not balanced by its hydration enthalpy.
Question 5.
How would you account for the irregular variation of ionisation enthalpies (first and second) in the first series of the transition elements?
Answer:
Irregular variation of ionisation enthalpies is mainly attributed to varying degree of stability of different 3d configuration (e.g., d0, d5, d10 are exceptionally stable).
Question 6.
Why is the highest oxidation state of a metal exhibited in its oxide or fluoride only?
Answer:
Oxygen and fluorine have small size and high electronegativity. Hence, they can oxidise the metal to the highest oxidation state.
![]()
Question 7.
Which is a stronger reducing agent Cr2+ or Fe2+ and why?
Answer:
The following reactions are involved when Cr2+ and Fe2+ act as reducing agents.
![]()
The ![]() value is -0.41 V and
value is -0.41 V and ![]() value is +0.77 V. This means that Cr2+ can be easily oxidised to Cr3+, but Fe2+ does not get oxidised to Fe3+ easily. Therefore, Cr2+ is a better reducing agent that Fe2+.
value is +0.77 V. This means that Cr2+ can be easily oxidised to Cr3+, but Fe2+ does not get oxidised to Fe3+ easily. Therefore, Cr2+ is a better reducing agent that Fe2+.
Question 8.
Calculate the spin only magnetic moment of M2+ (aq) ion (Z = 27).
Answer:
Electronic configuration of M atom with Z = 27 is [Ar] 3d7 4s2.
∴ Electronic configuration of M2+ = [Ar]3d7, i.e.,
![]()
Hence, it has three unpaired electrons.
∴ Spin only magnetic moment (μ) = \(\sqrt{n(n+2)}\)
= \(\sqrt{3(3+2)}\)
= \(\sqrt{15}\)
= 3.87 BM
Question 9.
Explain why Cu+ ion is not stable in aqueous solutions.
Answer:
In aqueous solutions Cu+ undergoes disproportionation to form a more stable Cu2+ ion.
2Cu+(aq) > Cu2+(aq) + Cu(s)
The higher stability of Cu2+ in aqueous solutions may be attributed to its greater negative △hyd.H0 than that of Cu+. It compensates the second ionisation enthalpy of Cu+ involved in the formation of Cu2+ ions.
![]()
Question 10.
Actinoid contraction is greater from element to element than lanthanoid contraction. Why?
Answer:
In actinoids, 5f orbitals are filled. These 5f orbitals have a poorer shielding effect than 4f orbitals (in lanthanoids). Thus, the effective nuclear charge experienced by electrons in valence shells in case of actinoids is much more than that experienced by lanthanoids. Hence, the size contraction in actinoids is greater as compared to that in lanthanoids.
