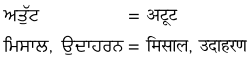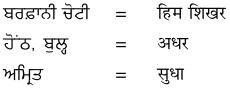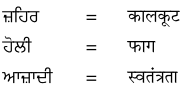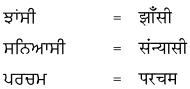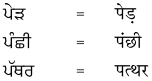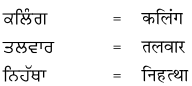Punjab State Board PSEB 7th Class Hindi Book Solutions Chapter 12 अशोक का शस्त्र-त्याग Textbook Exercise Questions and Answers.
PSEB Solutions for Class 7 Hindi Chapter 12 अशोक का शस्त्र-त्याग (2nd Language)
Hindi Guide for Class 8 PSEB अशोक का शस्त्र-त्याग Textbook Questions and Answers
अशोक का शस्त्र-त्याग अभ्यास
1. नीचे गुरुमुखी और देवनागरी लिपि में दिये गये शब्दों को पढ़ें और हिंदी शब्दों को लिखने का अभ्यास करें :

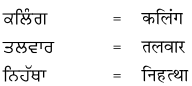
उत्तर :
विद्यार्थी अपने अध्यापक/अध्यापिका की सहायता से स्वयं करें।

2. निहत्था नीचे एक ही अर्थ के लिए पंजाबी और हिंदी भाषा में शब्द दिये गये हैं। इन्हें ध्यान से पढ़ें और हिंदी शब्दों को लिखें :

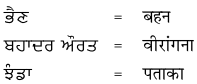
उत्तर :
विद्यार्थी अपने अध्यापक/अध्यापिका की सहायता से स्वयं करें।
3. इन शब्दों/मुहावरों के अर्थ लिखकर वाक्यों में प्रयोग करें :
- मंत्रमुग्ध होना ____________ _____________________________
- लोहा लेना ____________ _____________________________
- सदा के लिए आँखें बंद कर लेना ____________ _____________________________
- पूर्णाहुति ____________ _____________________________
- तलवार फेंक देना ____________ _____________________________
- सिर काटना ____________ _____________________________
- सिर न झुकना ____________ _____________________________
- सदावर्त ____________ _____________________________
- साक्षात् चंडी-सी दिखाई देना ____________ _____________________________
- मृत्यु की गोद में सो जाना ____________ _____________________________
- मुख पर चिंता की छाया होना ____________ _____________________________
उत्तर :
- मन्त्रमुग्ध होना – पूरी तरह मुग्ध होना।
वाक्य – सभी श्रोता गायक का गीत सुनकर मन्त्रमुग्ध हो गए।
- लोहा लेना – युद्ध करना, मुकाबला करना।
वाक्य – भारतीय सेना किसी भी शत्र से लोहा लेने को तैयार है।
- सदा के लिए आँखें बन्द कर लेना मर जाना।
वाक्य – वैभव के दादा जी नब्बे वर्ष के थे कि अचानक दिल का दौरा पड़ने से उन्होंने सदा के लिए अपनी आँखें बन्द कर लीं।
- पूर्णाहुति – यज्ञ की अन्तिम आहुति।
वाक्य – कल यज्ञ की पूर्णाहुति पड़ेगी।
- तलवार फेंक देना – पराजय स्वीकार करना।
वाक्य – महाराज अशोक ने तलवार फेंक दी और बौद्ध धर्म अपना लिया।
- सिर काटना – मार देना।
वाक्य – भगवान कृष्ण ने अत्याचारी राक्षस का सिर काटना उचित समझा।
- सिर न झुकना – हार न मानना।
वाक्य – रानी लक्ष्मी बाई ने अंग्रेज़ों के आगे सिर न झुकाया।
- सदावर्त – अखंड भोज।
वाक्य – महेश्वर ने घर में सदावर्त लगा रखा है।
- साक्षात चण्डी – सी दिखाई देना–निश्चित मृत्यु प्रदान करने वाली वीरांगना।
वाक्य – युद्ध में लक्ष्मीबाई साक्षात् चण्डी जैसी दिखाई देती थी।
- मृत्यु की गोद में सो जाना – मर जाना।
वाक्य – वृद्ध रोगी मृत्यु की गोद में सो गया।
- मुख पर चिंता की छाया होना – परेशान होना।
वाक्य – पुत्र की बीमारी के कारण पिता के मुख पर चिंता की छाया साफ दिख रही थी।

4. सिर काटना सिर न झुकना सदावर्त साक्षात् चंडी-सी दिखाई देना मृत्यु की गोद में सो जाना मुख पर चिंता की छाया होना उपयुक्त शब्द से रिक्त स्थान भरें :
(क) अशोक ने स्वयं सेना का …………………………… करने का निश्चय किया। (नेतृत्व, संचालन, स्वामित्व)।
(ख) कलिंग से युद्ध …………………………… चलता रहा। (तीन, पाँच, चार वर्ष)
(ग) कलिंग के महाराजा के मरने का समाचार पाकर अशोक …………………………… हुए। (प्रसन्न, विस्मित, स्तब्ध)
(घ) कलिंग के फाटक बंद हैं, यह समाचार सुनकर अशोक …………………………… हुए। (लज्जित, दुःखी, उत्तेजित, क्रोधित)
(ङ) पद्मा के सम्मुख अशोक तलवार फेंक देता है क्योंकि वह ……………………………। (डर, निराश, नारी वध नहीं करना चाहता था, आत्मग्लानि)
उत्तर :
(क) संचालन
(ख) चार वर्ष
(ग) प्रसन्न
(घ) उत्तेजित
(ङ) नारी वध नहीं करना चाहता था।
5. इन प्रश्नों के उत्तर एक या दो वाक्यों में लिखें :
(क) अशोक अपने शिविर में परेशान क्यों है?
उत्तर :
भयंकर नरसंहार तथा कलिंग को न जीत पाने के कारण अशोक अपने शिविर में परेशान थे।
(ख) संवाददाता ने अशोक को क्या समाचार दिया?
उत्तर :
संवाददाता ने सम्राट अशोक को कलिंग के महाराजा का युद्ध में मारे जाने का समाचार सुनाया।
(ग) मगध की विजय हुई है, पूछने पर संवाददाता चुप क्यों रह जाता है?
उत्तर :
सम्राट अशोक के यह पूछने पर कि क्या मगध की विजय हई थी? संवाददाता इसलिए चुप हो जाता है क्योंकि कलिंग दुर्ग के फाटक अभी भी बन्द था। फिर किस मुँह से वह कहता कि कलिंग जीत लिया गया है।
(घ) अशोक ने सेना-संचालन का भार अपने ऊपर क्यों लिया?
उत्तर :
कलिंग दुर्ग के फाटक खुलवाने के लिए सम्राट अशोक ने सेना संचालन का भार अपने ऊपर ले लिया।

(ङ) अशोक पद्मा के युद्ध के लिए ललकारने पर तलवार क्यों फेंक देता है ?
उत्तर :
पद्मा द्वारा युद्ध के लिए ललकारने पर भी अशोक तलवार इसलिए फेंक देता है। क्योंकि वह कहता है कि मैं स्त्री – वध नहीं करूँगा।
(च) बदला लेने का अवसर मिलने पर भी पद्मा अशोक को युद्धभूमि से सुरक्षित क्यों जाने देती है?
उत्तर :
सम्राट अशोक ने पदमा के समक्ष सिर झुका कर कहा कि काट दो इस सिर को। मैं अपराधी हूँ। पद्मा ने कहा जाइए महाराज ! स्त्रियाँ भी निहत्यों पर वार नहीं करेंगी।
6. इन प्रश्नों के उत्तर-चार या पाँच वाक्यों में लिखें :
(क) सम्राट अशोक ने बौद्ध धर्म क्यों अपनाया?
उत्तर :
सम्राट अशोक ने कलिंग पर विजय प्राप्त करने के लिए उस पर आक्रमण कर दिया। भीषण युद्ध हुआ। लाखों लोग मारे गए। कलिंग के महाराजा भी मारे गए। फिर भी कलिंग के दुर्ग का फाटक बंद था। भीषण रक्तपात ने सम्राट अशोक के मन को बदल दिया। इस बदलाव के कारण सम्राट अशोक ने बौद्ध धर्म स्वीकार कर लिया।
(ख) सम्राट अशोक ने बौद्ध भिक्षु के सामने क्या-क्या प्रतिज्ञाएँ की?
उत्तर :
सम्राट अशोक ने बौद्ध भिक्षु के सामने निम्नलिखित प्रतिज्ञाएँ की
- जब तक मेरे शरीर में प्राण हैं, अहिंसा ही मेरा धर्म होगा।
- मैं सबसे प्रेम करूँगा और मेरी करूणा का सदाव्रत आप सबको मिलेगा।
- मैं आजीवन अपनी प्रजा की भलाई करूँगा।
- सब धर्मों को समान दृष्टि
- सब प्राणियों को सुख और शान्ति पहुँचाने का प्रयत्न करूँगा।
7. लिंग बदलें :
- सम्राट = सम्राज्ञी
- देवी = ………………………..
- महाराज = ………………………..
- परुष = ………………………..
- प्रति = ………………………..
उत्तर :
- सम्राट = सम्राज्ञी
- देवी = देवता
- महाराज = महारानी
- पुरुष = स्त्री
- पति = पत्नी।

8. समुचित विराम चिह्नन लगायें :
यह कौन है क्या साक्षात् दुर्गा कलिंग की रक्षा करने के लिए युद्ध भूमि में उतर आई है शेष सैनिक भी सभी स्त्रियाँ हैं क्या स्त्रियों से युद्ध करना होगा क्या अशोक को स्त्रियों का भी वध करना होगा ना ना मैं स्त्री वध नहीं करूँगा मुझे विजय नहीं चाहिए मैं यह पाप नहीं करूंगा मैं शस्त्र नहीं चलाऊँगा।
चिन्तन
(1) बुद्धं शरणं गच्छामि।
अर्थात् बुद्ध की शरण में जाता हूँ।
(2) संघं शरणं गच्छामि।
अर्थात् संघ (संस्था) की शरण में जाता हूँ।
(3) धर्मं शरणं गच्छामि।
अर्थात् धर्म की शरण में जाता हूँ।
मनन : सर्वप्रथम गुरु की शरण, फिर संस्था, फिर धर्म। गुरु एक व्यक्ति है: व्यक्ति से बड़ी संस्था है, सब से ऊपर धर्म है। यहाँ संस्था का अर्थ संगठन, जमात, मत है। और धर्म का अर्थ मूल सिद्धांतसत्य, अहिंसा आदि हैं : जिसका स्थान सबसे ऊँचा है।
तुम प्रण करो कि जननी जन्मभूमि को पराधीन होते देखने से पहले तुम सदा के लिए अपनी आँखें बंद कर लोगी।
नारी (पदमा) की यह घोषणा देश प्रेम का ज्वलंत उदाहरण है। नारियों में वीरता, त्याग व बलिदान की भावना मनुष्यों से कम नहीं, उपरोक्त कथन पर मनन करें। देश प्रेम का व्रत लें। पद्मा जैसी हठव्रती, त्यागमयी, वीर नारी ने ही हिंसक अशोक को अहिंसक और करुणामय बना दिया। इतिहास को नयी दिशा दी।
उन नारियों के नाम पता करो जिन्होंने पद्मा के समान देश के लिए अपने-आप को पूर्ण रूप से समर्पण किया। ऐसी पुस्तकें पुस्तकालय से लेकर पढ़ें और उनकी जीवन से प्रेरणा लें।
प्रयोगात्मक व्याकरण
- कलिंग के फाटक आज बंद हैं।
- महाराज! आप यहाँ बैठिए।
- सैनिक ने अपनी तलवार झटपट संभाल ली।
- अधिक मत बोलो।
उपर्युक्त पहले वाक्य में ‘आज’ शब्द क्रिया के काल, दूसरे वाक्य में ‘यहाँ’ शब्द क्रिया के स्थान, तीसरे वाक्य में ‘झटपट’ शब्द क्रिया की रीति तथा चौथे वाक्य में ‘अधिक’ शब्द क्रिया की मात्रा संबंधी विशेषता बता रहे हैं अत: ये क्रिया विशेषण हैं।
अतएव क्रिया की विशेषता बताने वाले शब्दों को क्रियाविशेषण कहते हैं।

1. -मैं युद्ध कल करूंगा।
इस वाक्य में ‘कल’ शब्द से क्रिया के काल (समय) का पता लग रहा है। अतः यह कालवाचक क्रियाविशेषण है।
अतएव जो शब्द क्रिया के काल (समय) संबंधी विशेषता बताये, उसे कालवाचक क्रिया विशेषण कहते हैं।
अन्य कालवाचक शब्द:-रोज़, प्रातः, परसों, अभी, सुबह, शाम, रात, कभी, अब, तब, आजकल आदि।
2. सब आश्चर्य से उधर देखने लगते हैं। इस वाक्य में ‘उधर’ शब्द से क्रिया के स्थान का पता चल रहा है। अत: यह स्थानवाचक क्रियाविशेषण है।
अतएव जो शब्द क्रिया की स्थान संबंधी विशेषता बताये, उसे स्थानवाचक क्रियाविशेषण कहते हैं।
अन्य स्थानवाचक क्रियाविशेषण : यहाँ, वहाँ, इधर, ऊपर, नीचे, भीतर, बाहर, दूर, आगे, पीछे, चारों तरफ आदि।
3. वह बहुत बोलता है।
इस वाक्य में बहुत’ शब्द से क्रिया की मात्रा या परिमाण का पता चल रहा है। अत: यह परिमाणवाचक क्रिया विशेषण है।
अतएव जो शब्द क्रिया की परिमाण संबंधी विशेषता बताये, उसे परिमाणवाचक क्रियाविशेषण कहते हैं। अन्य परिमाणवाचक शब्द: थोड़ा, ज्यादा, कम, पर्याप्त, तनिक, इतना, उतना, न्यून, लगभग, काफी आदि।
4. संवाददाता महाराज से धीरे-से बोला।
इस वाक्य में ‘धीरे-से’ शब्द से क्रिया की रीति (ढंग) का पता चल रहा है अतः यह रीतिवाचक क्रिया विशेषण है।
अतएव जो शब्द क्रिया की रीति संबंधी विशेषता बताये, उसे रीतिवाचक क्रियाविशेषण कहते हैं।
अन्य रीतिवाचक क्रियाविशेषण : ऐसे, कैसे, जैसे, तैसे, वैसे, जल्दी-जल्दी, अकस्मात, अचानक, सहसा, सामान्यतः, साधारणतः आदि।
अशोक का शस्त्र-त्याग Summary in Hindi
अशोक का शस्त्र त्याग एकांकी का सार
इस एकांकी में सम्राट अशोक का शास्त्र त्याग तथा बौद्ध धर्म में दीक्षा लेने का वर्णन किया गया है।

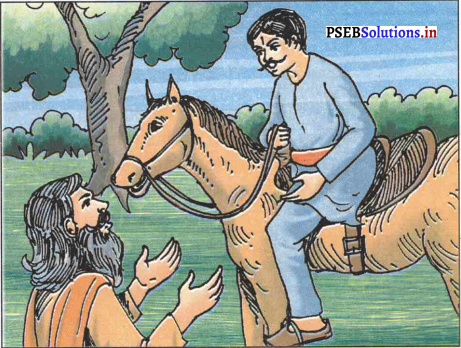
पिछले चार वर्ष से सम्राट अशोक की सेना कलिंग से लड़ रही थी। दोनों ओर के लाखों लोग मारे जा चुके थे, लाखों घायल हो चुके थे। फिर भी सम्राट अशोक कलिंग पर विजय नहीं पा सके थे। तभी संवाददाता खबर लाया कि कलिंग नरेश मारे गए। सम्राट अशोक ने समझा कि युद्ध में मेरी विजय हो गई पर पता चला है कि कलिंग दुर्ग के फाटक अभी भी बन्द थे। सम्राट अशोक ने उत्तेजित होकर निर्णय लिया कि कल वे स्वयं युद्ध का संचालन करेंगे या तो कलिंग जीत लेंगे या फिर मगध की सेना वापस चली जाएगी।
अगले दिन सम्राट अशोक ने सेना की कमान खुद सँभाली और अपने सैनिकों को उत्साहित करते हुए या तो कलिंग जीत लेने या फिर मौत के मुँह में सो जाने का आह्वान किया। तभी सहसा कलिंग के दुर्ग का फाटक खुला तथा कलिंग नरेश की लड़की पद्मा वीरांगना के वेश में स्त्रियों की सेना के साथ बाहर आई। उसने भी अपनी सेना को हत्यारे अशोक के विरुद्ध जी – जान से लड़ने का आह्वान किया।
स्त्रियों की सेना सम्मुख देख अशोक उन पर शस्त्र नहीं उठा सके। पद्मा ने पूछने पर बताया कि वह अपने पिता के हत्यारे अशोक से लड़ने आई थी। सम्राट अशोक अपराधी की भाँति पद्मा के सामने खड़ा हो गया कि वह स्त्रियों पर शस्त्र नहीं उठाएगा क्योंकि यह शास्त्र की आज्ञा थी। पद्मा ने पूछा कि निरपराधियों की हत्या करना किस शास्त्र में लिखा था? लज्जित होकर सम्राट अशोक सदा के लिए तलवार फेंक दी तथा पद्मा के सामने नतमस्तक होकर उससे अपना बदला चुका लेने के लिए कहा। पद्मा ने भी निहत्थे पर वार नहीं किया और वापिस दुर्ग में अपनी सेना के साथ चली गई।
सम्राट अशोक ने बौद्ध धर्म स्वीकार कर लिया और आजीवन युद्ध न करने और अहिंसा का पालन करने की शपथ ली।
अशोक का शस्त्र-त्याग कठिन शब्दों के अर्थ
- शिविर = छावनी।
- पताका = झण्डा।
- सम्राट् = राजा।
- संध्या = सायं।
- स्वतः = अपने आप।
- असफल = सफल न होना।
- संवाददाता = सन्देश लाने – ले जाने वाला।
- प्रसन्नतापूर्वक = खुशी से।
- विजय = जीत।
- कलिंग दुर्ग = कलिंग का किला।
- उत्तेजित = गुस्से में आना।
- संचालन = चलाना।
- शस्त्र सुज्जित = हथियारों से लैस।
- आत्मसमर्पण = अपने आपको सौंपना।
- शपथ = सौगन्ध।
- अधिकार = हक।
- सहसा = अचानक।
- वीरांगना = बहादुर स्त्री।
- चकित = हैरान।
- हत्या = मारना।
- जननी = माता।
- पराधीन = परतन्त्र दूसरे के अधीन।
- वध = हत्या।
- द्वन्द्व युद्ध = अकेले से अकेले की लड़ाई।
- भीषण = भयंकर।
- पूर्णाहुति = अन्तिम आहुति।
- आक्रमण = हमला।
- अटल = न टलने वाली।
- अहिंसा = हिंसा न करना।
- करुणा = दया।

अशोक का शस्त्र-त्याग गद्यांशों की सप्रसंग व्याख्या
1. मेरे वीर सैनिको ! आज चार साल से युद्ध हो रहा है, फिर भी हम कलिंग को जीत नहीं पाये हैं। उसके किसी दुर्ग पर मगध की पताका नहीं फहरा रही है। कलिंग के महाराज मारे गये हैं। उनके सेनापति पहले ही कैद हो चुके हैं, फिर भी कलिंग आत्मसमर्पण नहीं कर रहा है। आओ, आज हम अपनी मातृभूमि की शपथ लेकर प्रण करें कि या तो हम कलिंग के दुर्ग पर अधिकार कर लेंगे या सदा के लिए मृत्यु की गोद में सो जायेंगे।
प्रसंग – प्रस्तुत गद्यांश हमारी हिन्दी की पाठ्य पुस्तक में संकलित एकांकी ‘अशोक का शस्त्र – त्याग’ से लिया गया है। लेखक ने यहाँ सम्राट अशोक के द्वारा कलिंग पर किए गए आक्रमण एवं उसके परिणाम का सजीव चित्रण किया है।
व्याख्या – लेखक कहता है कि कलिंग के दुर्ग पर अधिकार जमाने के लिए सम्राट अशोक अपने वीर सैनिकों से कहता है कि आज चार वर्ष से कलिंग से उनका युद्ध चल रहा है लेकिन अभी तक वे कलिंग को जीत नहीं पाए हैं। कलिंग के किसी भी किले पर मगध की विजय पताका नहीं फहरा रही है। कलिंग नरेश को तो हमने मार दिया है। उसके सेनापति को हमने पहले ही अपनी कैद में कर रखा है, लेकिन फिर भी जाने क्यों कलिंग की जनता एवं सैनिक आत्मसमर्पण नहीं कर रहे हैं। वे हथियार नहीं डाल रहे हैं। इसलिए ये मगध के वीर सैनिक आज हम अपनी मातृभूमि की कसम खाते हुए प्रतिज्ञा करते हैं कि या तो हम कलिंग के किले पर मगध की विजय पताका फहरा देंगे या फिर अपने प्राणों की आहुति दे देंगे।
विशेष –
- सम्राट अशोक द्वारा अपने वीर सैनिकों का उत्साह बढ़ाने का चित्रण किया गया है।
- भाषा शैली प्रवाहमयी है।

2. सहसा दुर्ग का फाटक खुल जाता है। सब आश्चर्य से उधर देखने लगते हैं। उनकी तलवारें खिंची की खींची रह जाती हैं। शस्त्र – सज्जित स्त्रियों की विशाल सेना फाटक के बाहर निकलने लगती है। सेना के आगे पुरुष भेष में एक वीरांगना है, जो सैनिक भेष में साक्षात् चंडी – सी दिखाई देती है। यह कलिंग महाराज की लड़की पदमा है। स्त्रियों की सेना अशोक की सेना से कुछ दूरी पर रुक जाती है। अशोक के सिपाही मन्त्रमुग्ध से देखते रह जाते हैं। अशोक भी चकित रह जाते हैं।
प्रसंग – प्रस्तुत गद्यांश हमारी हिन्दी की पाठ्य पुस्तक में संकलित एकांकी ‘अशोक का शस्त्र – त्याग’ नामक शीर्षक से लिया गया है। लेखक ने यहाँ कलिंग युद्ध में हुए भीषण संहार का सजीव चित्रण किया है। यहाँ लेखक ने कलिंग नरेश पुत्री पद्मा को रणक्षेत्र में स्त्री सैनिकों के साथ रणक्षेत्र में आते हुए दिखाया है।
व्याख्या – लेखक कहता है कि सम्राट अशोक और उसकी सेना अभी कलिंग के किले पर अधिकार जमाने की सोच रहे थे कि अचानक कलिंग के किले का फाटक खुल जाता है। सभी मगध सैनिक हैरानी से इधर – उधर देखने लगते हैं। मगध सैनिकों की तलवारें उनकी मयानों से खींची की खींची रह जाती हैं। वे देखते हैं कि अस्त्र – शस्त्र लिए कलिंग की विशाल स्त्री – सेना किले के बाहर आ रही है।
सेना के आगे – आगे पुरुष का वेष धारण किए हए एक वीरांगना चल रही थी जो देखने में साक्षात माँ दुर्गा लग रही थी। वह वीरांगना कोई और न होकर कलिंग नरेश की पुत्री पद्मा थी। किले के द्वार से बाहर आकर पद्मा की स्त्री – सेना सम्राट अशोक की सेना से कुछ दूर पहले ही रुक जाती है। मगध के वीर सैनिक कलिंग की स्त्री सेना को मन्त्रमुग्ध से देखते रह जाते हैं। सम्राट अशोक भी इस प्रकार सुसज्जित स्त्री सेना को देखकर हैरान थे।
विशेष –
- लेखक ने कलिंग के रणक्षेत्र में पद्मा तथा उसकी वीरांगनाओं का चित्रण किया है।
- भाषा सरल, सहज तथा भावानुकूल है।

3. बहनो ! तुम वीर कन्या, वीर – भगिनी, और वीर – पत्नी हो ! मुझे तुमसे कुछ नहीं कहना। जिस सेना ने तुम्हारे पिता, भाई, पुत्र और पति की हत्या की है, वह तुम्हारे सामने खड़ी है। आज उसी से तुम्हें लोहा लेना है। तुम प्रण करो कि जननी जन्म – भूमि को पराधीन होते देखने से पहले तुम सदा के लिए अपनी आँखें बंद कर लोगी।
प्रसंग – प्रस्तुत गद्यांश हमारी हिन्दी की पाठ्य पुस्तक में संकलित एकांकी ‘अशोक का शस्त्र त्याग’ से अवतरित है। लेखक ने यहाँ कलिंग नरेश पुत्री पद्मा द्वारा वीर कन्याओं को अपनी जन्म – भूमि के लिए न्योछावर हो जाने के लिए प्रेरित करते हुए दिखाया है। –
व्याख्या – लेखक कहता है कि कलिंग नरेश की पुत्री पदमा स्त्रियों के अन्दर उत्साह का संचार करते हुए कहती है कि हे बहनो ! तुम वीर पिता की पुत्री हो, वीर भाइयों की बहनें हो और पति की पत्नी हो। तुमसे तुम्हारी इस राजकुमारी पद्मा को ज्यादा कुछ नहीं कहना है। बस इतना ही कहना है कि जिस सेना ने तुम्हारे पिता, भाई, पत्र तथा पति की हत्या की है, वह सेना तुम्हारे सामने खड़ी है। आज तुम्हें उनसे मुकाबला करना है। तुम सभी प्रतिज्ञा करो कि अपनी मातृभूमि को दासता की जंजीरों में देखने से पहले तुम सभी अपने देश की रक्षा में अपने प्राणों को न्योछावर कर देगी।
विशेष –
- लेखक ने राजकुमारी पद्मा के देश प्रेम तथा नेतृत्व करने की क्षमता को दर्शाया है।
- वाक्य – विन्यास सटीक है।
- भाषा सरल तथा सहज है।
4. मैं कलिंग महाराज की कन्या हूँ। मैं हत्यारे अशोक की सेना से लड़ने आई हूँ, जब तक मैं हूँ मेरी ये वीरागनाएँ हैं, कलिंग के भीतर कोई पैर नहीं रख सकता। कहाँ हैं अशोक, कहाँ हैं मेरे पिता का हत्यारा ? मैं द्वन्द्व – युद्ध करना चाहती हूँ।
प्रसंग – प्रस्तुत गद्यांश हमारी हिन्दी की पाठ्य पुस्तक में संकलित एकांकी ‘अशोक का शस्त्र त्याग’ से अवतरित है। लेखक ने अपने इस लेख में कलिंग युद्ध का चित्रण किया है। यहाँ लेखक ने वीरांगना पद्मा की वीरता एवं साहस को दर्शाया है।
व्याख्या – लेखक कलिंग नरेश की पुत्री पद्मा की वीरता एवं साहस का परिचय देते हुए कहता है कि राजकुमारी पद्मा पुरुष वेष में सम्राट अशोक सेना पर साक्षात् दुर्गा बनकर टूट पड़ती है वह अपना परिचय देते हुए कहती है कि वह कलिंग के महाराज की पुत्री पद्मा है। वह अपने पिता के हत्यारे अशोक और उसकी सेना से युद्ध करने के लिए आई है।
वह अशोक और उसकी सेवा को चुनौती देते हुए कहती है कि जब तक उसके प्राणों में प्राण हैं और कलिंग की वीरांगनाएं हैं तब तक मगध का कोई भी सैनिक कलिंग के अन्दर प्रवेश नहीं कर सकता। वह सम्राट अशोक पुकारती है और पूछती है कि उसके पिता कलिंग नरेश का हत्यारा अशोक कहाँ है। वह उसके साथ आमने – सामने का युद्ध करना चाहती है।

विशेष –
- लेखक ने राजकुमारी पद्मा की वीरता एवं अदम्य साहस का परिचय कराया है।
- भाषा प्रवाहमयी है।
![]()
![]()
![]()
![]()
![]()

![]()
![]()