Punjab State Board PSEB 5th Class Welcome Life Book Solutions Chapter 8 Self-Defence Textbook Exercise Questions and Answers.
PSEB Solutions for Class 5 Welcome Life Chapter 8 Self-Defence
Welcome Life Guide for Class 5 PSEB Self-Defence Textbook Questions and Answers
(a) Through the game of Karate
Self-defense means protection of oneself. If anybody attacks you how will you defend yourself from that attack?

Children, do you know, in last few days girls were given training of Karate in the school? It was given so that girls can defend themselves after learning Karate. Never depend on anybody.

Oral Questions:
Question 1.
What is self-defense?
Answer:
The meaning of self-protection is to protect ourselves.
Question 2.
Have you seen a game of Karate?
Answer:
Yes, I have seen.
Question 3.
Why Karate is needed?
Answer:
Karate is the art of fighting with hands without using any weapon. We do not keep weapons all the time with us and so there is a need to learn Karate for using hands as weapons.
Teacher will encourage other children for self-defense by action of some girls.
(b) Through the game of Fencing
Fencing is a very traditional game of the world. In old days many battles were won on the basis of swordsmanship. This game needs very hard work to be perfect. The ground of fencing is called “piste”. This game is also played on indoor ground or outdoor ground.

Both the swordsmen attack each other and use shield for their defence. You must have watched the skill/art of fencing in the serials like “The Ramayana’and The Mahabharataon T.V.

Oral Questions:
Question 1.
What is called the ground of fencing?
Answer:
The ground of swordplay is called Piste.
Question 2.
Where did you see fencing?
Answer:
In the programmes of T.V. like Ramayana and Mahabharata.
Question 3.
Which thing is used to defend attack of fencing?
Answer:
For this, shield is used.
(c) Through the game of “Gatka’/stick fighting
With the help of a poster, Teacher will tell the students how you can defend yourself during the game of Gatka and swordsmanship, It will be told to the students in simple way.

Teacher will tell that for sports where you require sportsmanship, you also need self -confidence and these achievements can not be attained without self¬confidence.
(Gatka is the art of war of Punjabis in which it is art of fighting against enemy during cease fire. Any male/female can take its training. Nihang Sikhs are perfect in this art. Art of weapon is one of the unlimited arts. Gatka is the one of these styles which is played in Punjab and many parts of Northern India. Highlights of this can be seen on HolaMohalla at Anandpur Sahib. Game of Gatka is a game of self-defence as similar to Japan’s game Karate. A Small shield and 3.5 palm long stick is used in Gatka (stick-fighting).

Oral Question:
Question 1.
Where have you seen the game of Gatka or stick-fighting?
Answer:
On the occasion of Hola-Mohalla at Shri Anandpur Sahib.
Question 2.
Who can play the game of Gatka or stick-fighting?
Answer:
Any man or woman can play this sport.
(d) Good Touch, Bad Touch
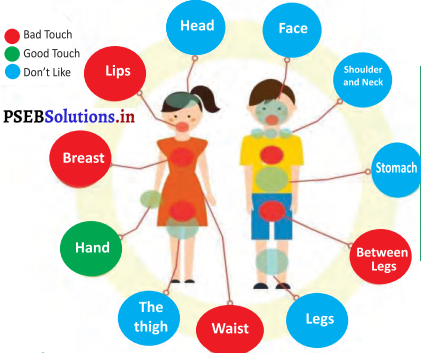
Teacher will give information to the students about good-bad touch with the help of the poster of body parts.

Oral Questions:

Question 1.
How do you feel when anybody touches you?
Answer:
If some of our family members touches us then we feel good but if some outsider touches us, we feel bad and get angry also.
Question 2.
What will you do when anybody touches you?
Answer:
We will try to prevent ourselves from him and will also inform our parents.
Question 3.
How do you deal in the market/ fairs?
Answer:
It is required to visit market/fairs very carefully so that nobody can come near us. They can touch us with bad intention and can steal our things also. So there is a need to be careful by ourselves and should remain with our parents.

(e) Conversation
Teacher : “Doyougoto the market /fairs or festivals and religious places?
Girls : “Yes, madam.
Teacher : “You must collide with each other at such places?
Girls : “There is a lot of scrimmages while watching glimpses.
Teacher : “There are molesters at such places who tease or molest girls, young girls or women.
Girls : (They are listening quietly while looking at teacher’s face).

Teacher : “If someone touches you except your parents, how do you feel?
Girls : “(Answer collectively)” Very bad.
Teacher : “Well done ! If you feel bad then that touch is not good then what should do?
Girls : All Quiet.
Teacher : “Never be frightened. Do not get nervous also. Tell your parents and start shouting. Do you understand my point?
Oral Question :
Question 1.
Do you go to see glimpses?
Answer:
Yes, a number of times.
Question 2.
Should you trust on strangers?
Answer:
No, not at all.

Question 3.
What will you do if someone touches you?
Answer:
We will tell our parents and create noise.
Teacher will interact with girls in a friendly environment and guide how to protect oneself from bad touch of anybody.
(f) Well Done! Minni
Mini reads in class 5. She is the monitor of the class. She is a good Kho- Kho player. In school, children play games like Kho-Kho and Kabaddi. Boys and girls play together in it.

It was Sunday.The Ramayana serial was going on. Mini was sitting quietly in front of the TV. Mother asked, “Mini is anything hurting?”
“No, Mom “while switching off the TV, Mini answered in a quiet voice.

“Then why is my butterfly sitting withered today?” Mother put her hand on her shoulder and said.
“Mom tell me! “After thin king for a while, Mini said, “Yes, say my love!” Mom said holding her hand lovingly!

“Now you put your hand on my shoulder then nothing happened.” Mini was talking intermittently as if remembering something.
(Mummy was looking surprisingly at her face)
“Yesterday while playing, when a boy put his hand on my shoulder, I didn’t like it. And after that I couldn’t play.” Minnie looked at her mother for answer.
This was the reason for your silence. The mother hugged Minnie and said, “My queen daughter, when I touch you, whether it is your shoulder, mouth or hand, nothing happens to you because I am your mother. You know this touch since your childhood So you like it.
Oh mom! Minnie said hugging. “Dear Minnie! Do you feel bad when a stranger touches you at these places!” The mother asked by looking at Minnie.
“Too much!” Minnie made a bad face and said, “Yes, dear! That means that touch is not good, it’s bad”. Mom taught,” Dear Minnie, if any touch that doesn’t feel good, makes you uncomfortable, is bad. If someone touches you in a bad way, tell your teacher, Father or me, but don’t be silent.
“Don’t worry mother! Your Mini is not so weak to let go the wrong toucher, as lama player” Mini said. She said laughingly while switching on T.V. “Well done Minnie!” The mother said taking Mini in her lap.

Oral questions :
Question 1.
Do you listen to stories from your elders?
Answer:
Yes, a number of times.
Question 2.
Which game do you play in the school?
Answer:
Kho-Kho and Kabaddi.
Question 3.
Do you support at home?
Answer:
Yes.
Question 4.
Which serial do you watch on TV?
Answer:
Ramayana.
(The teacher will inspire the girls with a story.)
(G) Protection from Pandemic
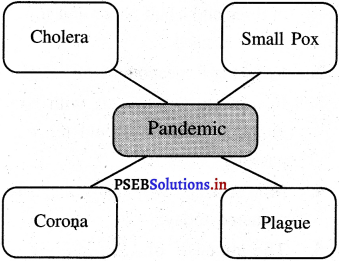
Any disease that affects people from different countries all over the world is called pandemic. For example Plague, Chicken pox, Cholera and Corona etc. Nowadays, Corona has turned into a pandemic.

Teacher will tell the children about Corona virus its symptoms and remedies in detail.
★ Teacher will explain this disease by an activity.For example, make a circle of the children. The children will stand behind each other. The circle will be filled by children.
★ Teacher will ask a child to push. When the child will push another child then the child standing in the front will fall. Thus, all the students standing in the circle will fall down.
(Teacher will tell that Corona is a viral disease and spreads from person to person. Teacher will also tell about prevention of it.)

Teacher will tell the children again to stand in a circle and repeat the same activity. The child will push again. All the children standing in circle will fall down.
Teacher will pick out a child from the circle holding his/her arm. Now children will stop falling.
(Teacher will tell that in this disease social and physical distancing is a must otherwise, this chain will continue. Teacher will tell that in order to break this chain we should have distance from others, wear mask, cover mouth with cloth or handkerchief while sneezing, keep your surroundings clean and wash hands for at least 20 seconds.)

 Wear mask
Wear mask
 Wash hands properly multiple times
Wash hands properly multiple times
 Keep physical/ social distancing
Keep physical/ social distancing
Oral Question :
Question 1.
Do you know about pandemics?
Answer:
Yes, with this a number of people get sick in a very short period. I know the name of that disease – Corona.
Question 2.
Tell me the name of any pandemic?
Answer:
Yes, with this a number of people get sick in a very short period. I know the name of that disease – Corona.

Question 3.
What can we do to be safe from COVID-19?
Answer:
Cover your nose and mouth with masks, wash your hands again and again, keep social distancing at least 1 kilometre far away from each other. Keep your surroundings clean.
PSEB 5th Class Welcome Life Guide Self-Defence Important Questions and Answers
Multiple Choice Questions :
Question 1.
How can we do self-defence?
(a) Karate
(b) Swordplay
(c) Gataka
(d) All are correct.
Answer:
(d) All are correct.
Question 2.
What do we call the ground of swordplay?
(a) Piste
(b) Shield .
(c) Astroturf
(d) None of the above.
Answer:
(a) Piste
Question 3.
The correct statement is :
(a) Self-defence means to protect ourselves.
(b) Swordplay is the oldest sport in the world.
(c) Gataka is a fatal art.
(d) All are correct.
Answer:
(d) All are correct.

Question 4.
The wrong statement is :
(a) Nihang Singhs are experts in the art of Gataka.
(b) Only men can learn Gataka.
(c) Gataka is played in Punjab.
(d) Karate is a weapon-art of Japan.
Answer:
(b) Only men can learn Gataka.
Question 5.
The weapon-art of Karate is related to which country?
(a) Japan
(b) India
(c) Kerala
(d) Norway
Answer:
(a) Japan.
Question 6.
Which of the following is pande-mic?
(a) Cholera
(b) Plague
(c) Corona
(d) All are correct.
Answer:
(d) All are correct.

Question 7.
What should we do to protect ourselves from Corona?
(a) To wear masks
(b) To wash hands again and again
(c) Keep Social distancing
(d) Adopt all the above measures.
Answer:
(d) Adopt all the above measures.
Question 8.
Which game is related to self-defence?
(a) Carom Board
(b) Karate
(c) Hockey
(d) None of the above.
Answer:
(b) Karate.
Fill in the blanks :
1. In Gataka, a of the length …………………………………… of three and a half hands and small shield is used.
2. Karate is a weapon-art of ………………………………….. .
3. If someone …………………………………… us with bad intention then we should tell our parents.
4. Mother asked why her …………………………………… is withered today.
5. The disease of …………………………………… is a pandemic.
Answer:
1. long stick
2. Japan
3. touches
4. butterfly
5. Corona.

Tick Right (✓) or Wrong (✗) :
1. If someone touches us with bad intention then we should create noise.
2. We should not shake hands with one-another to protect ourselves from Corona.
3. Mini was the monitor of fifth class.
Answer:
1. ✓
2. ✓
3. ✓
Mind Mapping :
Answer:
Match the following :
1. Karate – (a) Punjab
2. Gataka – (b) Pandemic
3. Corona – (c) Piste
4. Swordplay – (d) Japan
Answer:
1. (d)
2. (a)
3. (b)
4. (c).

Short Answer Type Questions
Question 1.
Tell the names of some weapon- arts.
Answer:
Karate, Swordplay, Gataka.
Question 2.
Gataka is the art of which place?
Answer:
Gataka is played in Punjab and some areas of North India.
Question 3.
What is used to play Gataka?
Answer:
A long stick of three and a half hands and small shield is used.
Question 4.
Where is the sport of swordplay played?
Answer:
Swordplay can be played in the open ground or closed ground.
Question 5.
What should we need to do to avoid corona pandemic?
Answer:
Wear mask, wash hands again and again, mention distance from each other, don’t shake hands with others, don’t touch the someone things.

Question 6.
If someone touches you with bad intention, then what would you do?
Answer:
We will tell our parents and create noise.
![]()
![]()






















 Wear mask
Wear mask Wash hands properly multiple times
Wash hands properly multiple times Keep physical/ social distancing
Keep physical/ social distancing